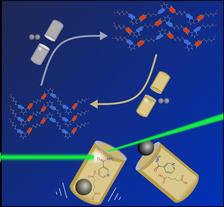
The result of mechanochemical synthesis can be altered simply by selecting different milling jars and balls. Using the bright X-ray light from PETRA III (shown in green), the team was able to follow the formation of different polymorphs live. (Credit: McGill University, Luzia Germann)
The physical properties of milling jars and balls used in mechanically driven chemical reactions have a considerable influence on the reaction mechanism and outcome. Achieved at PETRA III, this is the result of a time-resolved X-ray study of mechanochemical syntheses. It shows that the material of milling jars, as well as the size and material of the milling balls can be specifically used to control the results of mechanochemical co-crystallisations, as Luzia S. Germann from McGill University (Canada) and co-workers report in the Royal Society of Chemistry's journal Chemical Science.
Mechanochemistry has recently gained a lot of attention as a cornerstone of green and environmentally-friendly solvent-free synthetic methods. The results of the synchrotron X-ray powder diffraction experiments will contribute to a better understanding of mechanochemical processes and how they can be used in the future to explore the synthesis of new materials.
“In recent years, mechanochemistry has proven to be an important ‘green’ means of synthesising compounds, whereby mechanical energy drives the desired chemical reaction,” explains Germann who is currently a postdoctoral fellow with the group of Tomislav Friščić at McGill University. In this technique, the reactants for the desired material are usually placed in a milling jar together with milling balls made for example out of steel and shaken for a while. This grinding process drives the synthesis in the jar.
“This technique is particularly important when it comes to ‘Green Chemistry’, because this method of synthesis does not require long reaction times, high temperatures, toxic starting materials, or large amounts of solvents,” says Germann. “On top of this, this procedure generally produces less waste products in addition to better control of the reaction.” Mechanochemical syntheses are often used to synthesise new compounds and materials, including so-called pharmaceutical cocrystals, binary molecular compounds containing an active pharmaceutical ingredient (API).
Small amounts of liquids are often added during the milling process to speed up the reaction, but also to screen for new potential phases of the desired molecules, so called polymorphs that have the same chemical composition but different properties due to an altered arrangement of the molecules in solid-state – like diamond and graphite. “Our work shows that it is not just the external conditions like grinding additives that play an important role in the reaction, but also the reaction vessel itself,” reports co-author Martin Etter from DESY, who is in charge of the Powder Diffraction and Total Scattering beamline P02.1 at PETRA III where part of the experiments were conducted.
„Mechanochemical transformations have been known and in use since the dawn of humanity, but their mechanisms have remained mostly a mystery until the introduction of synchrotron radiation technologies available at highly advanced research facilities like DESY,“ notes Friščić.
Among other things, the team investigated the synthesis of cocrystals made up of the active pharmaceutical ingredient nicotinamide (C6H6N2O) and adipic acid (C6H10O4). They used X-rays to observe the formation of different cocrystals in real time during the reaction. “The time-resolved measurements at PETRA III were of great importance for our research, because they allowed us to monitor the formation of the different crystalline phases and correlate them with the different milling conditions,” says Germann.
The observations came as a surprise to the researchers. “By using different milling jars, we were able to make different polymorphs, which are compounds having the same chemical composition but different configurations in the solid-state,” explains Germann. “Milling jars were previously seen as simple containers where the reaction takes place. Our work shows how important the choice of material can be. We were able to switch between different polymorphs simply by using a different milling jar, independent of any grinding additives. This phenomenon is surprising and has never been observed for organic materials and cocrystals. This may change the way how we think about mechanochemistry in organic reactions and may change the way how we plan experiments in the future.”
The size and material of the milling balls used also had an effect on the reaction product. The researchers attribute this to a change in the efficiency with which energy is transferred between the milled materials. “To the best of our knowledge, this work represents the first demonstration how a mechanochemical reaction can be directed to produce either thermodynamically stable or metastable cocrystal polymorphs, only by changes to the milling jar and balls,” the team writes. This is of importance for synthesis and screening of new materials, for example pharmaceutical cocrystals containing an API, using mechanochemistry.
The work was carried out by scientists from McGill University in Montreal, the Max Planck Institute for Solid State Research in Stuttgart, the University of Warsaw, and DESY.
(from DESY News)
Reference:
Challenging the Ostwald rule of Stages in Mechanochemical Cocrystallisation; Luzia S. Germann, Mihails Arhangelskis, Martin Etter, Robert E. Dinnebier and Tomislav Friščić; Chemical Science, 2020; DOI: 10.1039/d0sc03629c