The synthesised Na3(N2)4 in a reaction chamber of a diamond anvil cell. (Credit: DESY)
Nitrogen, the most abundant element in the Earth’s atmosphere, is an inert and stable substance which rarely reacts with other materials at ambient conditions. Still, the compounds of nitrogen are important ingredients of our everyday life, like for instance: light emitting diodes, hard coatings, fertilisers. One of the pathways to stabilise novel exciting nitrogen-containing materials has opened through reactions at very high pressures and temperatures. In the study, which has been published in the journal Inorganic Chemistry, an international team of researchers, also from DESY, used sodium azide NaN3 as a precursor to synthesise novel sodium-nitrogen compounds at extreme conditions. The products of this synthesis were characterised by means of single-crystal X-ray diffraction at the Extreme Conditions PETRA III beamline P02.2 at DESY. Some experiments were also performed at the Advanced Photon Source (APS) in Chicago (US).
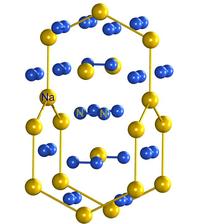
Compounds containing covalently-bound dinitrogen N···N groups are very common in biological and organometallic synthetic ambient pressure chemistry. They play an important role in the processes of nitrogen reduction to ammonia: one of the major industrial processes in the world. However, there are only a few extended solid-state materials containing such units. The particular feature of these compounds is that their composition is always MN2. M stands for an element, usually a metal in its standard oxidation state, while the nitrogen unit accommodates up to four electrons. Control of the element oxidation state, and therefore, the number of electrons x on the [N2]x- is the key to tune the material’s properties.
In this work the researchers compressed sodium azide NaN3 to ~28000 atmospheres in a diamond anvil cell and heated it by a near infrared laser to ~1900 K. At these extreme conditions NaN3 decomposes and produces a substance with a very unusual composition Na3N8 [or Na3(N2)4]. In its crystal structure, nitrogen atoms are connected with each other forming nitrogen-nitrogen dumbbells, therefore, the expected composition of this Na-N compound would be NaN2, where the [N2] unit receives one electron from the sodium atom. In Na3N8, however, each dinitrogen unit formally absorbs only 0.75 electrons.
This discovery demonstrates that dinitrogen anions can accommodate non-integer formal charges, and therefore, it opens a new class of inorganic materials. Moreover, further measurements also provide insights into the mechanisms of high-pressure reactions: compounds like Na3N8 may be quite common intermediates in various high-pressure chemical processes involving nitrogen. This study indicates that exploration of nitrogen and its chemistry at extreme conditions may trigger new developments in high-pressure chemistry and materials science.
Reference:
Dinitrogen as a Universal Electron Acceptor in Solid-State Chemistry: An Example of Uncommon Metallic Compounds Na3(N2)4 and NaN2. Maxim Bykov, Kelin R. Tasca, Iskander G. Batyrev, Dean Smith, Konstantin Glazyrin, Stella Chariton, Mohammad Mahmood, Alexander F. Goncharov; Inorganic Chemistry, 2020, DOI: 10.1021/acs.inorgchem.0c01863