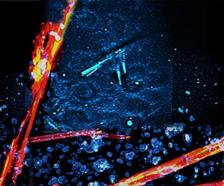
Among other things, the multimodal multiphoton microscope can reliably distinguish salt crystals (here in red) from protein crystals (here in blue) (Credit: Cheng, Q., Chung, H., Schubert, R. et al., Commun Biol).
A novel kind of microscope is able to detect tiny protein crystals, which are beyond the imaging power of even modern light microscopes. The innovative technique relies on various non-linear optical effects to image even nanocrystals, which are increasingly being used for protein structure analyses nowadays. The team that developed this method, headed by DESY’s Franz Kärtner, Christian Betzel from the University of Hamburg and Guoqing Chang from the Chinese Academy of Sciences, is presenting its “multimodal multiphoton microscope” in the journal Communications Biology.
The spatial structure of proteins tells scientists something about how these biomolecules work and function. This is important not only in order to understand the processes taking place inside organisms, but also for developing new medical drugs. The function of a protein can, for example, be specifically blocked using an active substance that is customised to bind to the active site of the biomolecule. To determine the structure of proteins, researchers usually grow small crystals of the biomolecules and shine X-rays through them. The crystals diffract the X-rays, producing a characteristic pattern at the detector. This diffraction pattern can be used to calculate the spatial structure of the crystal and hence that of its individual building blocks, the proteins.
The brighter and more tightly focused the X-rays, the smaller the crystals for structural analysis can be. This is a major advantage, because many proteins are very difficult to grow as crystals, since this is not a state that is meant to occur in their natural environment. In many cases, only tiny crystals of such proteins can be grown. “As X-ray sources are becoming better and better, there is an increasing demand for micro- and nanocrystals,” explains Betzel who, like Kärtner, is also a member of the Hamburg Centre for Ultrafast Imaging, CUI.
The problem facing the researchers is that they first have to find these tiny crystals in the suspension. The protein crystals are not only small, however; they are usually transparent and not unlike salt crystals. “For years, we have been coming up against the limits of our ability to detect these minute crystals, even using ultramodern microscopes,” explains Kärtner, who works at the Centre for Free-Electron Laser Science, CFEL, which is jointly operated by DESY, the University of Hamburg and the Max Planck Society. “These mostly colourless protein crystals are extremely difficult to locate in aqueous solutions when we are preparing to take measurements, and they are often overlooked altogether,” adds Qing-di Cheng, one of the lead authors and PhD student at University Hamburg.
The team of scientists therefore designed a special multiphoton microscope (MPM) for protein crystals. It combines two different effects to make the sensitive crystals visible. Firstly, it causes the crystals to fluoresce. Secondly, it makes use of a non-linear effect, whereby when a high-intensity laser beam is shone at these crystals, they also produce light at wavelengths of a half and a third that of the incoming radiation, so-called higher harmonics. The advantage is that in both cases the image seen under the microscope only lights up at the corresponding wavelength where a protein crystal is present, but not in its surroundings.
The new multiphoton microscope uses a specially designed multicolour fibre laser that generates intense ultrashort laser pulses in the infrared range lasting just one tenth of a trillionth of a second (tenth of a picosecond). These pulses initially have a wavelength of 1550 nanometres. By doubling the frequency, the system generates laser pulses with half that wavelength, 775 nanometres, which lies just beyond the visible spectrum in the near infrared. At the same time, the system also produces laser pulses with a wavelength of 1300 nanometres through non-linear wavelength conversion. The sample is illuminated by both these pulses.
“The multiphoton microscope driven by this type of a fibre-based ultrafast light source provides a robust and cost-effective means of detecting protein crystals using frequency doubling, frequency tripling, and three-photon fluorescence,” says Hsiang-Yu Chung, a postdoctoral fellow at CFEL and the other lead author of the publication.
The crystals are now made visible by three processes. The shorter wavelength pulses cause certain protein crystals to emit pulses of radiation at half their wavelength, the so-called second harmonic. In this case, they radiate in the ultraviolet (UV) range at 387.5 nanometres. The longer wavelength pulses, at 1300 nanometres, produce the third harmonic in most protein crystals, i.e. pulses with one third of the original wavelength. The resulting 433 nanometre radiation is at the blue end of the visible spectrum.
The shorter wavelength is also chosen such that it can trigger fluorescence in the amino acid tryptophane, which occurs in proteins. To do so, electrons in this amino acid need to absorb three photons (light particles) at once from the laser pulse. This is possible if the density of the laser photons is high enough. For this to be the case, the laser must be tightly focused. The multiple absorptions which are of interest and which give multiphoton microscopes their name, can only take place at the focal point. With the new protein microscope, the resulting fluorescence lies in the UV range, whereas the laser beam used is in the infrared range. This makes it easy to separate the light produced from the incoming light, so that the radiation coming from the protein crystals can be reliably detected.
The technique has been put to the test in the laboratory using suspensions containing various microcrystals. Tiny crystals of the proteins lysozyme, thaumatin, thermolysin and PAK4 were reliably detected. “Commercially available microscopes for such applications are limited in their performance; in particular, they are much less sensitive and cannot reliably distinguish between protein crystals and salt crystals,” says Chang. “The MPM system described by us is likely to attract a great deal of interest within the structural biology community, especially in laboratories working at X-ray sources, but also at institutions and laboratories working in neurobiology, for example.”
The research was supported by the Cluster of Excellence “Advanced Imaging of Matter” of the German Research Foundation (DFG) – EXC 2056 – Project ID 390715994, the Helmholtz Excellence Network “Structure, Dynamics and Control on the Atomic Scale”, the Helmholtz Young Investigator Group (VH-NG-804), the Helmholtz-CAS Joint Research Group (HCJRG 201), the DFG project BE1443/29-1, as well as by the German Aerospace Centre (DLR) and the BMBF via the projects 50WB1422,05K16GUA. The Joachim-Herz Stiftung Hamburg also supported this research via the Infecto-Physics project.
(from DESY News)
Reference:
Protein-crystal detection with a compact multimodal multiphoton microscope; Qing-di Cheng, Hsiang-Yu Chung, Robin Schubert, Shih-Hsuan Chia, Sven Falke, Celestin Nzanzu Mudogo, Franz X. Kärtner, Guoqing Chang & Christian Betzel; Communications Biology, 2020; DOI: 10.1038/s42003-020-01275-8