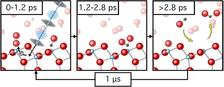
Using FLASH, the research team was able to precisely resolve that between 1.2 and 2.8 picoseconds after triggering, the carbon monoxide oxidised to carbon dioxide (picture: DESY Nanolab).
An international team of scientists have studied, for the first time, the light induced oxidation of carbon monoxide (CO) to carbon dioxide (CO2) on the surface of an oxide photocatalyst in real-time using the soft X-ray free-electron laser FLASH combined with theoretical calculations. According to their findings, which are published in the journal ACS Catalysis, the photoconversion of CO to CO2 takes place between 1.2 and 2.8 picoseconds (ps) after the reaction is triggered by an ultrafast optical laser pulse.
Photocatalysts are materials that can promote chemical reactions triggered by light. They are at the edge of industrial application. 'Photocatalysts have an array of potential applications including air and water purification and self-cleaning surfaces. In order to ensure their efficient use and to optimise their performance, it is essential to understand the early stage photodynamics on the active photocatalyst surface', explains Heshmat Noei, project leader from the DESY NanoLab.
Titanium dioxide (TiO2) – one of the most promising candidates for industrially applicable photocatalyts – was now scrutinised by the research group with the ultrashort laser pulses of the FLASH free-electron laser (FEL). Following exposure of TiO2 to a mixed atmosphere of carbon monoxide (CO) and oxygen (O2), the team synchronised an optical laser of 770 nanometres wavelength to the femtosecond X-ray laser pulses from FLASH. This optical light pulse triggered the photocatalytic oxidation of carbon monoxide while the FLASH pulses were used to study the reaction dynamics on the surface in real time. 'Despite the high importance of understanding early-stage reaction dynamics in photocatalysis, detailed information is lacking. Utilising the superconducting RF accelerator technology at FLASH, we were able to directly follow the photoreaction in real-time on a picosecond time scale', explains Michael Wagstaffe, postdoctoral researcher at the DESY NanoLab and the first author of the manuscript.
Using soft X-ray photoemission spectroscopy, individual chemical compounds can be identified with high surface sensitivity. By collecting a series of photoemission spectra recorded at specific times after the photoreaction was triggered, the team was able to monitor the evolution of the photoemission spectra and observe the transient formation of new chemical compounds. Their results led them to postulate that within the first 1.2 picoseconds after light illumination the oxygen activation process takes place, in which an electron is transferred from the titanium oxide surface to an oxygen molecule adsorbed on the surface. Between 1.2 and 2.8 picoseconds after irradiation they observed CO2 on the surface via a new component in the photoemission spectra, resulting from the oxidation of CO. 'With the help of the ultra-short FEL pulses, we were able to directly visualise the photoreaction and in doing so were able to determine reaction time scales and clarify that no long-lived intermediate species were formed', explains Wagstaffe.
Theoretical calculations carried out by a collaborative group of scientists at the Bremen Centre for Computational Materials Science (BCCMS) of Bremen University and the Max Planck Insititute for Structure and Dynamics of Matter predicted that oxygen adsorption on the photocatalyst surface leads to the formation of a so-called charge transfer complex. This means that the reaction can be initiated following the direct transfer of electrons from TiO2 to physisorbed O2 following laser illumination at a photon energy of 1.6 eV (770 nm). 'Our study shows that, according to the proposed mechanism, it is also possible to trigger the photoreactions on TiO2 with visible light – rather than the conventional way which requires ultraviolet light with shorter wavelengths', says Heshmat Noei.
In addition to the fundamental knowledge obtained, this study potentially opens up a new avenue of research, where the unique possibilities offered by soft X-ray free-electron lasers can be used to investigate the crucial first steps of photoreactions on the surfaces of catalysts which is one of the research topics studied at the Hamburg cluster of excellence CUI: Advanced Imaging of Matter.
(from DESY News)
Reference:
Ultrafast Real-Time Dynamics of CO Oxidation over an Oxide Photocatalyst; Michael Wagstaffe, Lukas Wenthaus, Adrian Dominguez-Castro, Simon Chung, Guilherme Dalla Lana Semione, Steffen Palutke, Giuseppe Mercurio, Siarhei Dziarzhytski, Harald Redlin, Nicolai Klemke, Yudong Yang, Thomas Frauenheim, Adriel Dominguez, Franz Kärtner, Angel Rubio, Wilfried Wurth, Andreas Stierle and Heshmat Noei; ACS Catal. 2020, 10, 22, 13650–13658 DOI: 10.1021/acscatal.0c04098