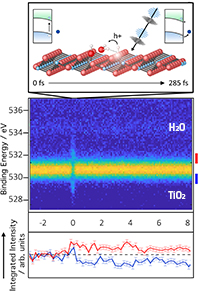
Drawing of the light-induced dynamics of water molecules on the titanium dioxide surface. Details: see figure below (Credit: Authors, DESY)
Efficient photocatalysts for solar-driven water splitting are very important for the green hydrogen-based economy. A full understanding of light-induced processes on the semiconductor photocatalysts in the presence of water molecules is therefore very relevant. An international team led by Heshmat Noei from the DESY NanoLab investigated the initial step of the complex reaction between water and a titanium dioxide (TiO2) surface. This promising photocatalyst is also important for a wide range of fields spanning chemistry, biology, environmental sustainability as well as for the development of technologies for air and water purification. The study was carried out at the free-electron laser FLASH and is published in the journal Physical Review Letters.
Rate-limiting steps of photocatalysts are difficult to measure on the relevant time scales. The phenomena are often very fast and taking place on a femtosecond time scale. “Our goal was to understand the light-induced water dynamics on a TiO2 surface by combining state-of-the-art ultrafast time-resolved photoemission spectroscopy at FLASH with theoretical calculations“, explains Heshmat Noei, scientist at DESY, “This allows us to probe the photoreaction at the interface between an oxide photocatalyst and water in real-time.”
The behavior of electron-hole pairs generated at the TiO2 photocatalyst surface following light illumination govern the efficiency of the entire photocatalytic process. They are highly reactive and responsible for a cascade of surface reactions. To optimise the performance of the device, it is crucial to breakdown the complex process and fully understand the individual reaction steps. This includes the influence of the adsorption state of the water molecules before illumination, the role of surface hydroxide species and a full mechanistic picture of what happens after illumination with all species involved. “By combining the ultra-short soft X-ray pulses of the FLASH at the beamline PG2 with an optical laser (wavelength: 770 nm) to trigger the photoreaction, one can directly follow the initial reaction steps in real-time.”, clarifies the first author Michael Wagstaffe from DESY, “By varying the time difference between the two laser pulses, we were able to observe an 'electronic movie' of the fleeting chemical changes at the catalyst surface.”
With this method, the scientists observed a charge redistribution at the surface within 285 fs which was made possible by the formation of a new hydrogen bond between water molecules (H2O) and the TiO2 (101) surface. However, this was only observed at low water coverage of about one layer. Additional layers lead to further water-water interactions above the surface weaken the interaction with the surface and suppress the reaction process.
Accompanying theoretical simulations were part of a large collaborative effort to understand the underlying mechanisms. A joint working group of scientists from the Bremen Center for Computational Materials Science (BCCMS) at the University of Bremen and the Max Planck Institute for the Structure and Dynamics of Matter (MPSD) in Hamburg carried out simulations. Their results fully corroborate the experimental observations. "Theoretical findings based on Ehrenfest dynamics simulations clarify the femtochemistry of the water splitting reaction on TiO2, highlighting the ultrafast nature of the charge-transfer and the role of all water-related species at the surface" explains Adrian Dominguez-Castro, from BCCMS.
These findings show how the activity of the photoactive surface is determined by ultrafast interfacial hole transfer from TiO2 to adsorbed water. A detailed understanding of the hydrogen bonding of molecules on a surface as well as their complex reaction pathways are crucial for further developments of photocatalysts.
Reference:
Photoinduced Dynamics at the Water/TiO2(101) Interface, M. Wagstaffe, A. Dominguez-Castro, L. Wenthaus, S. Palutke, D. Kutnyakhov, M. Heber, F. Pressacco, S. Dziarzhytski, H. Gleißner, V. K. Gupta, H. Redlin, A. Dominguez, T. Frauenheim, A. Rubio, A. Stierle and H. Noei, Physical Review Letters, 130, 108001 (2023), DOI: 10.1103/PhysRevLett.130.108001