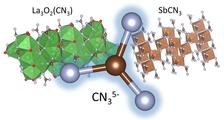
Crystal structures of La3O2(CN3) and SbCN3 featuring the same CN35- anion. (Credit: A. Aslandukov, University of Bayreuth)
Research groups from the University of Bayreuth, the University of Cologne and Goethe University Frankfurt simultaneously discovered the first representatives of guanidinates (SbCN3) and oxo-guanidinates (a series of Ln3O2(CN3) compounds where Ln = La, Eu, Gd, Tb, Ho, and Yb). Together with scientists from DESY, they conducted experiments at PETRA III at DESY. The international teams including researchers from the UK, France, Sweden and the U.S. also used the synchrotron sources ESRF in Grenoble (France) and the APS in Argonne (U.S.) for their research. These compounds, synthesised at high pressures and high temperatures, contain [CN3]5- anions, which are analogous to the well-known carbonate anions [CO3]2-, but each oxygen atom is replaced by nitrogen. This fundamental discovery extends the list of carbon-nitrogen inorganic anions, provides new opportunities for inorganic and organic synthetic chemistry and might be interesting for technological applications. The scientists reported their results in two papers published in the same issue of the scientific journal Angewandte Chemie.
Inorganic ternary metal-C-N compounds with covalently bonded C-N anions encompass important classes of solids. “The most investigated classes are cyanides (CN-) and carbodiimides (CN22-) which are used in a plethora of applications. While CN- and CN22- anions are well-known, the next member of this series — the CN35- anion, a completely deprotonated guanidine, has hitherto been unknown. Despite numerous attempts to synthesise gaunidinate anion by deprotonation of guanidine molecule using strong bases, up to date only partially deprotonated guanidine has been stabilised,” says Andrey Aslandukov from the University of Bayreuth.
In both studies the authors successfully stabilised CN35- guanidinate anion using not a classic “wet chemistry” approach but rather a non-conventional route of solid-state synthesis under extreme conditions in a laser-heated diamond anvil cell. “Using this technique, a sample can be compressed between two diamonds to several hundred thousand atmospheres and heated using a laser to temperatures reaching several thousand degrees Celsius,“ explains Konstantin Glazirin, a beamline scientist of P02.2 at PETRA III. Simultaneously, tightly focused X-rays of exceptionally high brilliance at this ‘Extreme Conditions Beamline’ allow tracking structural changes in the sample.
The SbCN3 and Ln3O2(CN3) compounds were synthesised in a diamond anvil cell from molecular nitrogen and antimony or molecular nitrogen and partially oxidised Ln. The scientists pressurised the precursors to more than 25 gigapascals (about 250,000 times the atmospheric pressure) and heated the samples to more than 2000 degrees Celsius. Carbon in the synthesised compounds originates from the surface of the diamond anvils.
Despite being synthesised under high pressure, the compounds remain stable at ambient conditions. “This stability offers potential for further upscaling the production and potential industrial applications. One of the seven discovered compounds, SbCN3 is a direct band-gap semiconductor, making it a promising candidate for optical devices. Additionally, our novel synthetic approach paves the way for other guanidinates (TCN3 (T=V, Nb, Ta) ) and ortho-nitridocarbonates (T'2CN4 (T' = Ti, Zr, Hf)), which are anticipated to be useful for photochemical water splitting and non-linear optical devices,” says Maxim Bykov from the Goethe University Frankfurt. “The synthesis of the new C-N anion is important not only for fundamental chemistry but may have far-going implications for planetology, as compounds containing such an anion may exist in the interior of exoplanets,” says Leonid Dubovinsky, professor at the University of Bayreuth.
Original publications:
Stabilization of the CN35- anion in recoverable high-pressure Ln3O2(CN3) (Ln = La, Eu, Gd, Tb, Ho, Yb) oxoguanidinates, A. Aslandukov, P.L. Jurzick, M. Bykov, A. Aslandukova, A. Chanyshev, D. Laniel, Y. Yin, F.I. Akbar, S. Khandarkhaeva, T. Fedotenko, K. Glazirin, S. Chariton, V. Prakapenka, F. Wilhelm, A. Rogalev, D. Comboni, M. Hanfland, N. Dubrovinskaia, L. Dubrovinsky, Angewandte Chemie International Edition (2023), DOI: 10.1002/anie.202311516
Stabilization of Guanidinate Anions [CN3]5- in Calcite-Type SbCN3, L. Brüning, N. Jena, E. Bykova, P. L. Jurzick, N. T. Flosbach, M. Mezouar, M. Hanfland, N. Giordano, T. Fedotenko, B. Winkler, I. A. Abrikosov, M. Bykov, Angewandte Chemie International Edition (2023), DOI: 10.1002/anie.202311519