Radiation damage in biological systems (Figure: From the original publication licensed under the Creative Commons Attribution License 4.0 (CC BY)).
A new international study has revealed insights into the mechanisms underlying radiation damage at the molecular level. The research, performed by an international team including scientists from DESY, was published in the journal Nature Chemistry. It reveals how extensive, localised water ionisation occurs due to the ultrafast processes triggered by core-level ionisation of solvated metal ions.
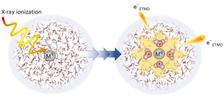
Radiation damage, particularly in biological systems, is primarily driven by the ionisation of water molecules and the subsequent formation of radicals and low-energy electrons. Instead of directly ionising biomolecules, this research demonstrates that the initiation of radiation damage is closely linked to solvated metal ions in water. This is a departure from previous theories that attributed radiation damage mainly to random events along the radiation path.
"A few years ago it was theoretically predicted that when X-rays are applied to magnesium ions, highly ionised and excited states can be formed, but they quickly relax with the help of surrounding water molecules, thus ionising them," says Eva Muchová from the University of Chemistry and Technology in Prague.
"In our publication, we demonstrate the detailed multi-step mechanism by which relaxation occurs using the example of solvated aluminum ions. It is clear that the relaxation of ions causes extensive and very localised ionisation of water, and we can talk about the formation of so-called 'hot-spots' of radiation damage," she adds.
The study specifically investigated the behavior of Al3+ ions in aqueous solutions after core-level ionisation. It was found that electronic relaxation in these systems involves two sequential solute-solvent electron-transfer-mediated decay processes.
According to Eva Muchová, one of the corresponding authors of the study, "The electron-transfer-mediated decay steps correspond to sequential relaxation from Al5+ to Al3+ accompanied by formation of four ionised water molecules and two low-energy electrons. Such charge multiplication and the generated highly reactive species are expected to initiate cascades of radical reactions."
The implications of this research are far-reaching, particularly in the context of radiation therapy for cancer treatment, where understanding radiation damage mechanisms is crucial for optimising treatment strategies. Additionally, this research could lead to new insights into the repair of DNA molecules and contribute to the development of more efficient radiation protection measures in various fields.
This study combined advanced experimental techniques, including liquid-jet photoemission spectroscopy, with high-level ab initio calculations to confirm the occurrence of these complex processes. All 1s ionization experiments were performed at the PETRA III beamline P04. The researchers emphasised that this work represents a significant step forward in our understanding of radiation damage at the molecular level and highlights the role of solvated metal ions in initiating these processes.
(Partly from News Fritz-Haber-Institut MPG, Berlin)
Reference:
G. Gopakumar, I. Unger, P. Slavíček, U. Hergenhahn, G. Öhrwall, S. Malerz, D. Céolin, F. Trinter, B. Winter, I. Wilkinson, C. Caleman, E. Muchová & O. Björneholm, Radiation damage by extensive local water ionization from two-step electron-transfer-mediated decay of solvated ions, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01302-1