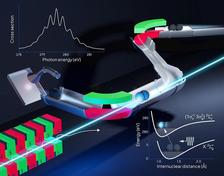
Schematic drawing of the photoabsorption experiment of negatively charged molecular carbon ions (C2-) at the PIPE end-station of the PETRA III beamline P04 (Credit: Elisa Monte, Justus-Liebig-Universität Gießen).
For the first time, vibrationally resolved inner-shell excitation of a negatively charged molecular ion was observed. A detailed quantum analysis of the measured data revealed a strong effect on the atomic distance. Charged molecular ions are of special interest, because they are components of flames, the Earth’s ionosphere, or interstellar gas clouds. The major challenge for the measurements, performed by researchers from the Justus-Liebig-Universität Gießen, Universität Hamburg, Goethe-Universität Frankfurt, and the Fritz-Haber-Institut in Berlin, has been the low particle density of the investigated C2- ions. Due to their electrostatic repulsion, this density is about a million times lower than that of electrically neutral molecules in photoabsorption experiments. Therefore, experiments with ions require a high photon flux and a high measurement sensitivity. These requirements are met by the photon-ion spectrometer at PETRA III (PIPE) at DESY. This unique instrument allows one to measure the transitions between different molecular vibrational states with a high resolution.
PIPE was set up at the PETRA III beamline P04 by a consortium of German research groups from the universities of Gießen, Hamburg, Frankfurt, Berlin (FU), and Kassel with funds from the German Federal Ministry of Education and Research (BMBF). The extremely high photon flux of up to several 1014 photons per second and a wide photon-energy range from 250 to 2600 eV offer worldwide unique conditions for photon-ion experiments at the beamline P04. In the PIPE setup, the ion beam from an ion source can be collinearly superimposed on the photon beam from the PETRA III light source along an interaction distance of 1.7 m in length.
The absorption of high-energy photons leads to the excitation or ionization of an electron from an inner atomic shell and thus to the formation of a highly excited molecular state. By emitting fluorescence photons or further electrons (Auger-Meitner effect), this state decays and is creating more highly charged molecular ions or fragments. They can finally be magnetically separated from the primary ion beam and individually detected with a highly sensitive detector.
In the present experiment with negatively charged carbon ions (C2-), the primary ions were generated with a cesium sputter ion source and accelerated to an energy of 6 keV. High-resolution photoabsorption measurements were carried out of this ion beam in the region of the carbon K edge with a photon-energy resolution of only 50 meV. The yield of positively charged molecular carbon ions (C2+) was measured as a function of the photon energy. On the basis of a detailed analysis of the resulting vibrationally resolved absorption spectrum, the molecular potential of the excited electronic intermediate state of the C2- molecular ion could be determined. Surprisingly, the distance between the two carbon atoms decreases significantly by almost 20% after excitation of the K shell. Such a drastic geometric effect, which should also be very relevant, e.g., for radiation biophysics, can also be expected after photoexcitation of other molecular anions. Moreover, the results obtained here may enable the detection of C2- in cold cosmic gas clouds. Further high-resolution measurements of photoexcitation of other small molecular anions are planned at the PIPE apparatus in the future.
Original publication:
Vibrationally Resolved Inner-Shell Photoexcitation of the Molecular Anion C2-; S. Schippers, P.-M. Hillenbrand, A. Perry-Sassmannshausen, T. Buhr, S. Fuchs, S. Reinwardt, F. Trinter, A. Müller, and M. Martins; ChemPhysChem 24, e202300061 (2023), DOI: 10.1002/cphc.202300061