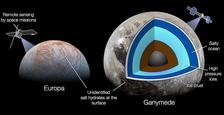
Jupiter's moon Ganymede has an underground ocean. (Credit: NASA/JPL-Caltech/SwRI/MSSS/Kevin M. Gill, CC BY-2.0 )
By reproducing the conditions of the hidden oceans within icy moons in our solar system, an international team including scientists from DESY led by Baptiste Journaux from the University of Washington, found two new kinds of salty ice. The results could help future exploration of icy moons like Jupiter's Ganymede. The scientists report their experiments at the European Synchrotron Radiation Source ESRF and at DESY in the Proceedings of the US National Academy of Sciences (PNAS).
Could there be life beyond Earth? Could moons host habitable environments and how did they evolve over time? Can we decipher the history of the solar system? What chemical processes take place in these icy moons?
Icy moons have a frozen surface primarily composed of water ice, or a mixture of water ice and other substances such as salts and organic compounds. These satellites are found in the outer solar system, beyond the orbit of Mars, and are thought to have formed from the accretion of icy particles and gas around their host planets. Examples include Europa and Ganymede, two moons of Jupiter, and Enceladus and Titan, two of Saturn's moons.
The space missions Galileo and Cassini-Huygens have greatly expanded our understanding of icy moons. In particular, they confirmed the presence of hidden oceans inside these bodies, based on liquid water underneath a crust of ice.
“These are the only planetary bodies other than Earth where liquid water is stable at geological timescales, which is crucial for the apparition and development of life,” explains Journaux. “They are, in my opinion, the best place in our solar system to discover extraterrestrial life, so we need to study their exotic oceans and interiors to better understand how they formed, evolved, and can retain liquid water in cold regions of the solar system, so far away from the Sun.”
Natural antifreeze
Presumably the water in these oceans contains table salt (sodium chloride; NaCl), which acts as a natural antifreeze, lowering the freezing point of water and allowing it to remain liquid at temperatures that would normally freeze pure water. However, planetary scientists had, until now, not been able to clearly identify it at the surface, despite using NASA space mission’s surface reflectance infrared spectroscopy. None of the compounds known would match the icy moon surface spectra.
Journaux explains the challenges: “Salt and water are very well known in common conditions. However, beyond that, we are totally in the dark. Now we have these planetary objects that probably have compounds that are very familiar to us, but at very exotic conditions. We have had to redo all the fundamental mineralogical science done in the 19th and early 20th century but at high pressure and low temperature.”
Due to these conditions, the water, which is thought to have a rich composition, crystallises, forming pure water ice and hydrates. When crystallised, it either sinks down to the mantle or goes up to the crust.
The international collaboration of scientists from the University of Washington, the ESRF, DESY, the Institute of Geochemistry and Petrology in Switzerland, the University of Bayreuth in Germany, NASA’s Jet Propulsion Laboratory, and the University of Chicago came to the ESRF and DESY to study these solid samples using X-ray diffraction. This method can reveal the inner structure of crystallised materials by shining X-rays on them and recording the way how they are diffracted by the sample.
“Our goal was to understand which compounds form in the high pressure and low temperature conditions of the icy moons,” explains DESY co-author Anna Pakhomova, who now works as a scientist at the ESRF. “In our experiments, we used single crystal X-ray diffraction to understand how the new hydrates are organized on atomic scale. It is a superior and unique technique to get unambiguousinformation on a solid’s crystal structure.”
To recreate the conditions of the icy moons, the scientists used a diamond anvil cell that compresses the sample between two tiny, but super hard diamonds and placed it in a cryostat, an elaborate scientific freezer, at the Extreme Conditions Beamline P02.2 of DESY's X-ray source PETRA III. There, they put the samples under up to 25 000 times the standard atmospheric pressure. The samples, which consisted of table salt dissolved in water, soon crystallized into previously unknown forms of salt hydrates.
Stable at the moons' surfaces
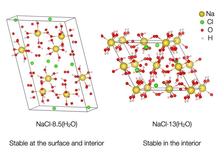
Until then, the scientific community had knowledge about the presence of just one hydrate, which is a compound that has very few water molecules trapped within its crystal structure. Its composition is a simple structure with one salt molecule for every two water molecules.
The new experiments unveiled two new different stable solid phases, both with a much higher amount of water in the structure and stable at different pressures and temperature. One has two sodium chlorides for every 17 water molecules; the other has one sodium chloride for every 13 water molecules. This would explain why the signatures from the surface of Jupiter’s moons are more “watery” than expected.
“These new phases are fascinating because they demonstrate a new diversity of water/salts crystal structures at high pressure and low temperature that wasn’t expected and remain to be explored for other compounds”, says Journaux.
The team was also able to show that one of the new salt hydrate, NaCl-8.5(H2O), is stable at the surface conditions of icy moons, and it should be the most common type of salt hydrate on icy worlds. “It has the structure that planetary scientists have been waiting for to explain the mysterious surface spectra of icy surfaces. This will permit us to identify where the best places on their surfaces are to explore, and eventually land and dig to look for signs of life,” explains Journaux.
The next step in this research is to study other salt species relevant for icy ocean worlds and measure their spectral properties to make sure they can be detected in upcoming space missions, such as the European Space Agency’s JUpiter ICy moon Explorer (JUICE), launching this April, and NASA’s Europa clipper, scheduled for 2024, both entering the Jupiter orbit in the early 2030s.
(from DESY News)
Reference:
On the discovery of hyper-hydrated sodium chloride hydrates, stable at icy moon conditions; Baptiste Journaux, Anna Pakhomova, Ines E. Collings, Sylvain Petitgirard, Tiziana Boffa Balaran, J. Michael Brown, Steve D. Vance, Stella Chariton, Vitali .B. Prakapenka, Dongyang Huang, Jason Ott, Konstantin. Glazyrin, Gaston Garbarino, Davide. Comboni, Michael. Hanfland; PNAS, 2023; DOI:10.1073/pnas.2217125120