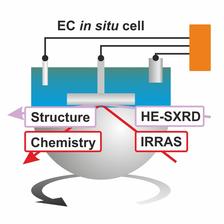
Combined IR spectroscopy and surface X-Ray diffraction setup for chemical and structural in-situ characterisation at the PETRA III beamline P07 (Credit: DESY).
Electrocatalysis is the decisive factor for a future energy system based on renewable resources. Typically, the most active electrocatalysts retain a high level of complexity in terms of structure and chemical composition. New analytical approaches are required to advance the characterisation of electrocatalysts under operating conditions. In particular, to correlate the structure with the reactivity of the active sites at the dynamically changing liquid/solid interface. An interdisciplinary team from the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) and DESY developed a versatile experimental setup.
It combines two complementary in-situ techniques for the simultaneous chemical and structural analysis of planar electrodes under electrochemical conditions (middle figure): high-energy surface X-ray diffraction (HE-SXRD) and infrared reflection absorption spectroscopy (IRRAS). The setup includes a three-electrode electrochemical IRRAS cell in the Fourier-transform infrared (FTIR) spectrometer with a hemispherical, IR-transparent CaF2 window. IR light enters through the IR-transparent window while the high energy X-ray beam enters directly through the cell walls optimized for low X-ray absorption. The diffracted high energy X-ray beam can easily penetrate the CaF2 window.
The research team tested the potential of the experimental setup by studying the oxidation of pre-adsorbed CO on a Pt(111) surface as well as the oxidation of the Pt(111) electrode itself. The study was carried out at the PETRA III beamline P07 and was published in The Journal of Physical Chemistry Letters.
This versatile setup allowed the researchers to obtain combined information about the interface structure and chemistry during the electrocatalytic reaction. The essential data were obtained by a simultaneous acquisition of the infrared reflection absorption spectra (IRRAS) (lower figure: (a) chemical information) and imaging of the reciprocal space with a 2D detector and high-energy X-rays ((b) and (c), structural information) at defined potentials. For the analysis of the surface structure and morphology of the Pt(111) electrode, the researchers analyzed the crystal truncations rods (CTR) which appear as vertical streaks (c). The analysis of the CTR intensity profiles (b) yields information on the lattice parameters, surface relaxations, and roughness of the Pt(111) (d).
With just a single experiment, the researchers were able to identify the adsorbates at the electrode surface, their adsorption geometries, the effect of the adsorbates on the surface morphology, and the structural evolution of Pt(111) during surface electro-oxidation. This versatile instrument in the DESY NanoLab will help advance the fundamental understanding of electrode materials in emerging technologies for energy conversion and storage.
Reference
O. Brummel, L. Jacobse, A. Simanenko, X. Deng, S. Geile, O. Gutowski, V. Vonk, Y. Lykhach, A. Stierle, J. Libuda, Chemical and Structural In-Situ Characterization of Model Electrocatalysts by Combined Infrared Spectroscopy and Surface X-ray Diffraction, J. Phys. Chem. Lett. (2023), DOI: 10.1021/acs.jpclett.3c01777