Hurlbutite crystals (centre) in the diamond anvil cell. The two crystals each measure roughly 0.01 millimetres across. (Credit: DESY, Anna Pakhomova)
In high-pressure experiments at DESY's X-ray light source PETRA III, scientists have observed a unique configuration of beryllium for the first time: At pressures nearly a million times the average atmospheric pressure, beryllium in a phosphate crystal acquires six neighbouring atoms instead of the usual four. This six-fold coordination had been predicted by theory more than 50 ago, but could not be observed until now in inorganic compounds. DESY scientist Anna Pakhomova and her collaborators report their results in the journal Nature Communications.
“Originally, chemistry textbooks stated that elements like beryllium from the second period of the periodic table could never have more than four neighbours, due to their electron configuration”, explains Pakhomova. “Then around 50 years ago theorists discovered that higher coordinations could actually be possible, but these have adamantly evaded experimental proof in inorganic compounds.” Inorganic compounds are typically those without carbon – apart from a few exceptions like carbon dioxide and carbon monoxide.
The scientists examined a rare natural mineral, hurlbutite, that is made of calcium, beryllium, phosphorus and oxygen (CaBe2P2O8). These phosphate crystals can naturally be found on Earth's surface. Using a so-called diamond anvil cell, the researchers applied high pressures on tiny natural hurlbutite crystals from Western Finland, provided by the University of Hamburg.
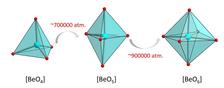
At ambient conditions, each beryllium atom in the crystal has four direct oxygen neighbours. The scientists increased the pressure stepwise and monitored the inner structure of the crystals between the small and ultra-strong diamond anvils with the bright X-rays of PETRA III at the Extreme Conditions Beamline P02.2. “At about 700,000 times the atmospheric pressure, the structure changes, giving beryllium a fifth neighbour”, reports Pakhomova.
Squeezing the crystals even further, their structure changed again at about 888,000 times the average atmospheric pressure, switching into a state in which beryllium has six neighbouring atoms. “To the best of our knowledge, these phases are the first examples of experimentally observed inorganic compounds possessing beryllium in a coordination higher than four”, says Pakhomova. With these observations, the study provides experimental proof for the theoretical predictions. Since our ambient conditions are extremely rare in the universe, such experiments also widen a general understanding of chemistry.
The University of Bayreuth, the Institute of Earth Sciences in St. Petersburg, the Materials Modeling and Development Laboratory in Moscow, the Linköping University and DESY participated in the work.
(from DESY news)
Reference:
Penta- and hexa-coordinated beryllium and phosphorus in high-pressure modifications of CaBe2P2O8; Anna Pakhomova, Georgios Aprilis, Maxim Bykov, Liudmila Gorelova, Sergey Krivovichev, Maxim P. Belov, Igor A. Abrikosov and Leonid Dubrovinsky; „Nature Communications“, 2019; DOI: 10.1038/s41467-019-10589-z