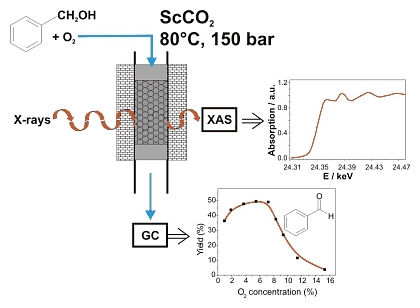
Figure 1: Strategy of the experiment for the in situ X-ray absorption spectroscopy studies during palladium-catalysed alcohol oxidation in "supercritical" carbon dioxide.
Jan-Dierk Grunwaldt, Matteo Caravati, Michael Ramin and Alfons Baiker
Department of Applied Chemistry and Biosciences, Swiss Federal Institute of Technology, Switzerland
Published in: Catalysis Letters 90, 221-229, 2003 and Catalysis Today 91-92, 1-5, 2004
Supercritical fluids have recently attracted considerable attention in catalysis, extraction, separation, crystallization, and polymer technologies due to their unique physical properties. Although they have led to better catalytic performance in a number of heterogeneously catalysed reactions, fundamental understanding is still lagging behind and necessitates in situ spectroscopic studies at high pressure. For the first time, a heterogeneous catalyst has been probed by X-ray absorption spectroscopy (XAS) in "supercritical" carbon dioxide (scCO2) under reaction conditions using a continuous-flow fixed-bed reactor. The catalytic activity was simultaneously determined by gas chromatography (GC).
"Supercritical" carbon dioxide as alternative solvent in catalysis
The use of "supercritical" carbon dioxide (scCO2) as solvent is attractive in catalysis for several reasons [3, 4]: Carbon dioxide is "supercritical" at rather moderate conditions (31 °C, 73.75 bar), relatively cheap, non-toxic and non-flammable. Moreover, it can be used as “green solvent” instead of organic solvents in hydrogenation and oxidation reactions, since it exhibits fair and tuneable solvent power for non-polar compounds [1-4]. As scCO2 is completely miscible with gases such as O2 and H2, the conventional gas-liquid reactions can be potentially performed in a single phase leading to better mass transport than in gas/liquid systems. In addition, product separation becomes often easier than in liquids and due to small reactor volumes processes can be intensified. The beneficial use of supercritical carbon dioxide has been demonstrated for several heterogeneously catalysed reactions [3, 5, 6]. Among them, oxidation reactions are interesting because of the chemical inertness of CO2 under oxidizing conditions [1, 2, 7-10].
Despite the fact that reactions often show increased rate or selectivity under "supercritical" conditions, fundamental studies are rare and there is strong need for suitable spectroscopic techniques [6]. Both unravelling the phase behaviour and monitoring of the solid catalyst under reaction conditions is mandatory. Here, focus is laid on the solid catalyst during the selective oxidation of benzylalcohol to benzylaldehyde over PdOx/Al2O3. X-ray absorption spectroscopy (EXAFS, XANES) is advantageous for such in situ studies since it allows to identify X-ray amorphous structures as encountered in catalysts and in situ cells can be constructed that mimic the conditions existing in real catalytic reactors [11-22].
Selective oxidation of benzylalcohol in supercritical carbon dioxide
The oxidation of benzyl alcohol to benzylaldehyde occurs over a 0.5wt% Pd/Al2O3 catalyst with high rate and selectivity (1800 molbenzaldehyde/molPdh at 80 °C and 150 bar, [1,2]). The rate is much higher than in organic solvents and 1 mg of finely dispersed palladium is sufficient to produce about 40 g benzaldehyde per day. Interestingly, the reaction rate strongly depends on the oxygen concentration in the feed as depicted in Figure 1. At high oxygen concentrations the catalytic performance is low. It can be speculated that palladium is oxidized on the surface leading to a lower activity. In order to prove this hypothesis, in situ studies are necessary.
For extending spectroscopic studies by X-ray absorption spectroscopy to supercritical fluids, an appropriate in situ spectroscopic cell was designed, which can be simultaneously used for X-ray absorption spectroscopy and catalytic studies (cf. principle shown in Figure 1). To warrant similar conditions as in the traditional continuous-flow fixed-bed reactor including optimal flow properties, the whole setup was kept as closely as possible to the laboratory experiment. Compared to the traditional reactor, the in situ spectroscopic cell is much smaller in size (volume 0.5 ml), in particular due to safety reasons, and is equipped with Be windows that withstand a pressure > 200 bar. Carbon dioxide is fed using a compressor, the alcohol with a liquid pump and gaseous oxygen by means of a dosing valve. The setup was installed at beamline X1 at ROEMO II (HASYLAB/DESY).
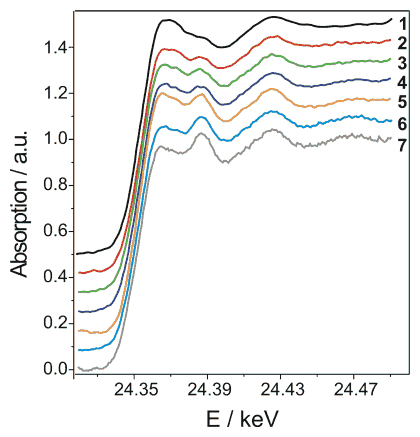
In the pursuit of probing the catalyst phase under supercritical conditions, we monitored the as-prepared catalyst in supercritical carbon dioxide at 80 °C and 150 bar and followed the structural changes during benzyl alcohol addition. The results, recorded in the continuous scanning mode (QEXAFS), are depicted in Figure 2. A fast reduction was observed in supercritical carbon dioxide above 70 °C. Actually, above this temperature a significant increase in reaction rate is observed, indicating that metallic Pd sites are crucial for the reaction.
Monitoring of structure and activity during alcohol oxidation in "supercritical" carbon dioxide
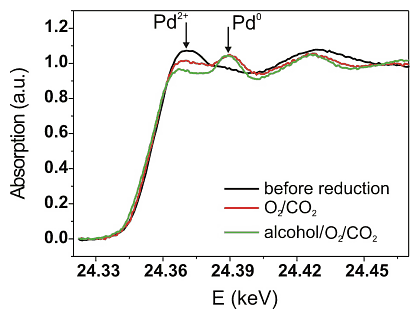
The next step was to uncover the oxidation state of the Pd/Al2O3 catalyst under reaction conditions. Both the catalytic activity and the X-ray absorption near edge structure at the Pd K-edge were determined simultaneously. Some of the results are given in Figure 3 and Table 1. The palladium constituent of the catalyst is mainly in metallic state under reaction conditions (1.9mol% benzyl alcohol, 0.95mol% oxygen in "supercritical" carbon dioxide). However, reconstruction of the spectra by linear combination with Pd0 and Pd2+ reference spectra showed that – in contrast to results in liquid phase [23, 24] – the palladium particles are partially oxidized under optimal reaction conditions. During increase of the oxygen concentration to 1.9 % an increase of the catalytic activity was observed, as expected from the previous laboratory experiments. The structure of the catalyst hardly changed. However, after oxidation of the palladium surface in 1.9%O2/scCO2 the reaction rate was lower and palladium more oxidized (Table 1). This supports that the deactivation of the catalyst at high oxygen concentrations is due to "overoxidation" of the Pd particles. A transient is observed in this last step and it takes some time until the catalytic activity reaches a steady state again. Linear combination analysis of the XANES data also revealed that the palladium particles are further reduced during this transient.
|
Experimental Conditions (in scCO2) |
Alcohol=0% O2=0% (after reduction) |
Alcohol=1.9% O2=0.95% |
Alcohol=1.9% O2=1.9% |
Alcohol=0% O2=1.9% |
Alcohol=1.9% O2=1.9% |
|---|---|---|---|---|---|
|
TOF (h-1) |
- |
1480 |
1890 |
- |
1280 |
|
Selectivity (%) |
- |
96.3 |
95.7 |
- |
94.4 |
|
Relative Pd0/Pd2+ ratio |
0.92/0.08 |
0.87/0.13 |
0.86/0.14 |
0.67/0.33 |
0.77/0.23 |
Schematic model for the alcohol oxidation in "supercritical" carbon dioxide
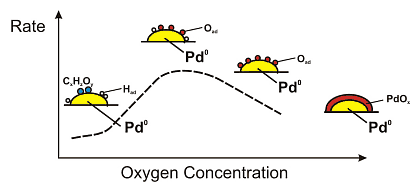
The oxidation of benzylalcohol can be assumed to occur in two steps. First the dehydrogenation of the alcohol to the corresponding aldehyde occurs and the formed hydrogen is adsorbed on the palladium particles. Then hydrogen is oxidized by the oxygen being present in the feed, which desorbs as water from the surface. The in situ obtained spectroscopic information indicate a direct relationship between the oxidation state of the palladium particles and their catalytic performance, as schematically shown in Figure 4. At low oxygen concentration, removal of adsorbed hydrogen originating from the dehydrogenation of the alcohol is slow. The oxidation reaction is thus more effective at higher oxygen concentrations in the feed. Furthermore, the better removal of carbonaceous species at higher surface oxygen coverage may play an important role. Further increase of the oxygen concentration leads, however, to a drop in activity due to "overoxidation" of the Pd particles. Metallic Pd surface sites are probably required for the activation of the dehydrogenation step.
Also recent corresponding EXAFS studies in liquid phase uncovered that a too high oxygen concentration leads to "over-oxidation" of the Pd catalysts and thus to a lower catalytic activity [23, 24]. Note however that the oxygen concentration can be much easier tuned in scCO2 and the oxygen tolerance is higher than in liquid phase, as presently investigated in more detail.
Acknowledgements
We want to take the opportunity to thank especially HASYLAB for supporting this work: In particular, M. Herrmann, P. Kappen at beamline X1, and the safety group at HASYLAB are gratefully acknowledged for their support and the constructive discussion of the safety aspects.
| References |
|
[1] J.-D. Grunwaldt, M. Caravati, M. Ramin and A. Baiker, Catal. Lett. 90 (2003) 221. |
| Contact information |
|
Jan-Dierk Grunwaldt (ETH Zürich) |
| Further Information |