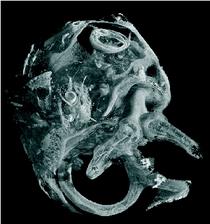
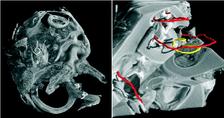
Figure 1: SRμCT was used to uncover the morphology of the temporal bone in cochlea with neural tube defects (left). The cochlea displayed the vestibular organ (foreground) and the fully developed 2.5 cochlear turns with an intact cochlear duct (background). The cochlear nerve with spiral ganglion neurons and nerve fibres showed a distinct contrast in SRμCT that allows for following neuronal tissue in the 3D dataset. The morphological analysis of the vestibulo-cochlear system in Pelizaeus Merzbacher disease is shown on the right. Based on the 3D dataset the centre lines (trajectories) of the cochlear duct (red) and of the Rosenthal canal (yellow) are represented together with the virtual cut lateral through the cochlea.
Felix Beckmann1, Joachim Schmutzhard2, Anita Lareida3,4, Rudolf Glückert2, Ilona Schwentner2, Irene Abraham2, Mario Bitsche2, Christina Falkeis5, Wolfgang Freysinger2, Consolato Sergi5, Annelies Schrott-Fischer2, Herbert Riechelmann2, and Bert Müller3,4
1. Institute for Materials Research, GKSS Research Centre, Max-Planck-Strasse 1, 21502 Geesthacht, Germany
2. Department of Otorhinolaryngology, Innsbruck Medical University, Anichstrasse 35, A6020 Innsbruck, Austria
3. Biomaterials Science Center, University of Basel, c/o University Hospital, 4031 Basel, Switzerland
4. Computer Vision Laboratory, ETH Zürich, Sternwartstrasse 7, 8092 Zürich, Switzerland
5. Institute of Pathology, Innsbruck Medical University, Austria
Published as:
“The cochlea in fetuses with neural tube defects”, Int. J. Devl. Neuroscience 27, 669-676 (2009).
“Pelizaeus Merzbacher disease: morphological analysis of the vestibulo-cochlear system”, Acta Oto-Laryngologica, 99999:1, (2009).
“High-resolution X-ray tomography of the human inner ear: synchrotron radiation-based study of nerve fibre bundles, membranes and ganglion cells”, Journal of Microscopy, Vol. 234, Pt 1, 95-102 (2009).
Microtomography using monochromatic synchrotron radiation (SRμCT) allows for the non-destructive, three-dimensional visualization with micrometer resolution and high contrast. The routine application of microtomography on different length scales at low and high photon energies allows one to characterize the complex anatomical structures of the human inner ear. The combination of SRμCT at DORIS III and conventional light microscopy after sectioning (histology) was used to uncover the morphology of the temporal bone in cochlea with neural tube defects and Pelizaeus Merzbacher disease. Parts of cochlea were carefully prepared using osmium tetroxide staining to visualize nerve fibre bundles and membranes in the two-and-a-half turn of the cochlea. Performing microtomography at PETRA III will give more detailed access to the interior structures of the human sensors down to subcellular level.
The hearing organ belongs to the most complex anatomical structures in the human body combining different types of delicate hard and soft tissues. Since already minor morphological deviations may result in crucial hearing deficiencies, the detailed knowledge of the human inner ear morphology is essential to achieve a better understanding of the inner ear pathologies (malformations), to improve the design and the insertion procedures of adapted cochlear implants as well as the treatment of hearing diseases. Imaging techniques allowing to simultaneously access the morphology of not only the complex bony structure, but also the soft tissue parts of the inner ear like membranes, nerve fibre bundles, ganglion cells are under ongoing developments. By now, neither clinical imaging techniques nor any kind of magnetic resonance imaging succeed within the required quality [1,2]. Currently, detailed morphological studies of the delicate microstructures such as sensory membranes, ganglion cells and nerve fibre bundles as parts of the inner ear exclusively rely on histological sectioning. By the sectioning, however, the soft tissues are mechanically deformed near the blade that often leads to damages of the thin membranes but at least to their relaxation to straight linear objects. This means that the complex anatomical structures of the inner ear can notably change their morphology, so that tissue shape and thickness can only be estimated [3]. Only microtomography using monochromatic synchrotron radiation on carefully prepared samples results in high detailed 3D datasets allowing to access the morphological structures within the cochlea. At the beamlines W2 (HARWI-II) and BW2 of the storage ring DORIS III SRμCT is routinely applied to small and large samples showing not only a high spatial resolution but also high density resolution in the tomograms [4]. Several bony structures and the implant bone interfaces within the human hearing organ for different types of implants were recently investigated using high-energy synchrotron radiation [5].
|
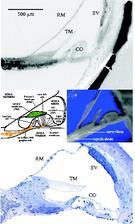
Within the current studies we used the combination of light microscopy and SRμCT to uncover the development and morphology of the vestibulo-cochlear system in temporal bone in Pelizaeus Merzbacher disease (PMD) and in cochlea with neural tube defects (NTD) (Fig. 1). PMD is a consequence of X-Linked mutation of the main central nervous system myelin protein resulting in a complex neurological syndrome. NTD are a frequent and heterogeneous group of malformations, ranging from the survivable spina bifida to fatal anencephaly. To increase the X-ray absorption of the soft tissues within the surrounding petrous bone, which is the hardest human tissue, osmium tetroxide staining was applied. This staining method, routinely applied to prepare tissues for electron microscopy, reacts with the unsaturated fatty acids and therefore essentially increases the X-ray absorption of cell membranes. Most of the petrous bone was removed to advance the spatial resolution of the tomographic data. For accessing the morphology of nerve fibre bundles and membranes low-energy SRμCT was applied to part of the cochlea (Fig. 2). The different delicate microstructures in the three-dimensional (3D) space was uncovered with isotropic sub-cellular resolution non-destructively and serve as complementary method to the classical histology of the inner ear. |
By the availability of the new stations for micro- and nanotomography at PETRA III the tomographical method will be boosted to access the interior structures of the human hearing organ down to cellular or even sub-cellular level. This will give new insights in the understanding of hearing diseases.
| References | ||||||||||
|
| Contact Information |
|
Felix Beckmann |