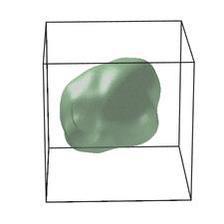
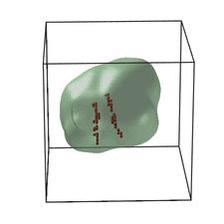
Schematic of an edge dislocation. (Copyright: Nature Energy (2018))
Researchers, also from DESY and led by a University of California San Diego team, have published work on cathode materials in the journal Nature Energy. The study, partly performed at PETRA III at DESY and the Advanced Photon Source APS in Argonne (US), explains what is causing the performance-reducing “voltage fade” of cathodes. This phenomenon currently plagues a promising class of cathode materials called Lithium-rich NMC (nickel magnesium cobalt) layered oxides.
These cathode materials have garnered considerable attention over the years as promising components for better rechargeable batteries for electric vehicles. The problem is, if this battery goes through series of charge-discharge cycles, its voltage fades and the amount of energy it can hold, and release later for use, also fades. The new research explains how this happens in Lithium-rich NMC cathode materials. In particular, the researchers identified nanoscale defects or dislocations in Lithium-rich NMC cathode materials as the batteries charged at a range of voltages going up to 4.7 volts.
“The dislocations are extra atomic layers that do not fit into the otherwise perfectly periodic crystal structure,” said Andrej Singer, the lead author who performed this work as a postdoctoral researcher at UC San Diego. “Discovering these dislocations was a big surprise: if anything, we expected the extra atomic layers to occur in a completely different orientation,” said Singer, a former PhD student from DESY, is now on the faculty at Cornell University. By combining experimental evidence with theory, the research team concluded that the nucleation of this specific type of dislocation results in voltage fade.
Knowing the origin of voltage fade, the team showed that heat treating the cathode materials eliminated most of the defects and restored the original voltage. They put the heat-treaded cathodes into new batteries and tested them at 4.7 volts, demonstrating that the voltage fade had been reversed. While the heat treating approach to reversing the defects is labor intensive and not likely to scale, the physics and materials science-based approach to characterizing and then addressing the nano-scale defects offers promise for finding new solutions to the voltage fade problem.
“Our paper is mainly about unlocking the mystery of the dislocations that cause voltage fade in Lithium-rich NMCs. We don’t have a scalable solution yet to solving the voltage fade problem in Lithium-rich NMCs, but we are making progress,” said UC San Diego nanoengineering professor Shirley Meng. She and UC San Diego Physics professor Oleg Shpyrko are the senior authors on the new Nature Energy paper.
“One of the most serious problems for lithium-rich NMC cathode materials is voltage fade.” said Minghao Zhang, a co-author on the paper. Voltage fade reduces the energy density of the battery, which in turn limits the practical applications of these materials despite their high energy density in the initial charge-discharge cycles.
For the first time the work clearly demonstrates that defect generation and defect accumulation in the structure of Lithium-rich NMC materials are the origin of voltage fade. Based on this explanation, the researches designed a heat treatment regime and then showed that the heat treatment removed the defects in the bulk structure and restored the battery output voltage.
Pinning Down Battery Details
The combined expertise allows the team to glean unprecedented insights of batteries while they are charging from X-ray imaging data gained from P10 beamline of PETRA III at DESY and the APS. “Being able to directly image the structure of materials and devices under operating conditions and with nanoscale resolution is one of the grand challenges in our quest to design and discover new functional materials,” said UC San Diego physics professor Oleg Shpyrko. “Our group’s efforts in developing novel X-ray imaging techniques are targeted towards fundamental understanding and ultimately control of defect formation. Our in-operando imaging studies indicate novel ways of mitigating voltage fade in next-generation energy storage materials.”
Looking to Solid State
The research described in the Nature Energy paper could eventually lead to new cathode materials for solid state batteries. Many researchers, consider solid state batteries to be one of the most promising future battery approaches. Lithium-rich NMC cathodes, for example, operate at high voltage and therefore could eventually be paired with solid state electrolytes, which also operate at high voltage. Solid state electrolytes are believed to be safer than the traditional liquid electrolytes used in Lithium-ion rechargeable batteries.
Reference:
Nucleation of dislocations and their dynamics in layered oxides cathode materials during battery charging, Nature Energy (2018) by A. Singer, M. Zhang, S. Hy, D. Cela, C. Fang, T. A. Wynn, B. Qiu, Y. Xia, Z. Liu, A. Ulvestad, N. Hua, J. Wingert, H. Liu, M. Sprung, A. V. Zozulya, E. Maxey, R. Harder, Y.S. Meng, O. G. Shpyrko, DOI: 10.1038/s41560-018-0184-2