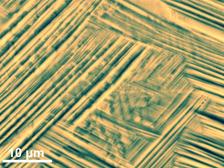
Scanning electron microscopy image showing the different phases in the peculiar gum-type titanium alloy. (Credit: Jian Zhang, MPIE)
Scientists from the Max-Planck-Institut für Eisenforschung (MPIE) have observed a new phase transformation in a titanium alloy at the X-ray source PETRA III at DESY. The mechanism they discovered could further our understanding of some surprising properties of certain alloys and be used to develop new materials. The team around main author Jian Zhang of the MPIE in Düsseldorf presents its findings in the journal Nature Communications.
The scientists used the light source PETRA III to examine the inner structure of a special alloy consisting of the (transition) metals titanium, niobium, tantalum and zirconium. This titanium alloy displays some unusual mechanical properties which have earned it the name “gum metal”. When mechanical stress is applied to the alloy, it behaves in a very interesting way: “On being deformed, it does not become harder or brittle, the way metals usually do, but instead it bends, almost like honey. In scientific terms, it has a very low elastic stiffness and very high ductility,” explains Dierk Raabe, director at MPIE, who co-authored the paper.
This makes the alloy extremely attractive for various industrial applications. In the aerospace industry, for example, it can be used as a kind of crash absorber. “When an aircraft’s turbine is damaged by hail or a bird strike, there is a risk that individual parts may shatter and damage the fuselage too. If parts of the protective casing around a turbine were made of this type of ‘gum metal’, they could capture the flying debris because the impact would not destroy but only deform them,” says Raabe.
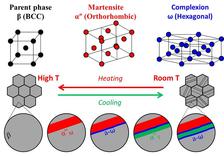
It is not yet quite clear why this alloy can be deformed to such a high degree. Various experiments have revealed peculiarities in its nanostructure, but have not yet shown a direct connection with its properties. Titanium alloys normally occur in two different phases, whereby the term phase refers to the crystal structure in which the atoms are arranged. At room temperature, the atoms are usually found in the so-called alpha phase, at high temperatures they switch to the beta phase. The metals display different properties, depending on which phase they occur in. Gum metals primarily consist of the beta phase, which is stable at room temperature in the case of these alloys.
The researchers at MPIE have now discovered a new mechanism during the phase transformation. The team of Jian Zhang has observed a new structure, which forms when the beta phase is transformed into the alpha phase: the omega phase. At DESY’s X-ray source PETRA III, the scientists were able to examine the crystal structure of the alloy in great detail during the transition. “When you shine X-rays onto a sample, the radiation is reflected by the crystal lattice. This produces a distinct pattern of reflections, a so-called diffractogram, from which we are able to deduce the relative positions of the atoms, in other words the crystal structure that they adopt,” explains DESY co-author Ann-Christin Dippel, who supervised the experiments technically and scientifically at the measuring station P02.1.
If the beta phase is cooled down rapidly from a high temperature, some of the atoms change position to adopt the energetically more favourable arrangement of the alpha phase. The movements of these atoms lead to mechanical stress along the phase boundary, almost as if the different phases were tugging on each other. When this stress exceeds a critical value, a new arrangement is adopted, the so-called omega phase.
“This newly discovered structure only arises when sheer stress is generated at the phase boundary, and it facilitates the transformation of the alpha into the beta phase. It can only exist between two other phases because it is stabilised by them,” reports Raabe. When the stress drops below the critical value because of the new layer, a new alpha phase layer is formed bordering on an omega phase. This results in a microstructure consisting of lots of layers, some of them on an atomic scale, each having a different structure. This transition also occurs when static forces are applied and is completely reversible. The scientists are now hoping that the newly discovered structure will help them to better understand the properties of this material and later to develop new, improved varieties of titanium alloys.
Xi'an Jiaotong University in China and the Massachusetts Institute of Technology in the USA were also involved in this research.
(from DESY News)
Reference:
Complexion-mediated martensitic phase transformation in Titanium; J. Zhang, C.C. Tasan, M.J. Lai, A-C. Dippel, D. Raabe; Nature Communications, 2017; DOI: 10.1038/ncomms14210