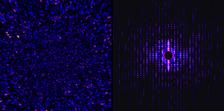
Left: A typical simulated incoherent diffraction pattern measured by the detector with seemingly random speckles. Right: After calculating the intensity correlations, the image resembles the pattern from coherent imaging and can be used to determine the structure. Credit: Kartik Ayyer, DESY
Physicists from the Friedrich-Alexander-University of Nuremberg-Erlangen (FAU) and DESY have worked out a new technique that could significantly improve the quality of X-ray analysis in crystallography compared to conventional methods. Incoherent diffractive imaging (IDI) could help to image individual atoms in nanocrystals or molecules faster and with much higher resolution, as the team reports in the journal Physical Review Letters.
For more than 100 years, X-rays have been used in crystallography to determine the structure of molecules. At the heart of the method is the principle of diffraction and superposition, to which all waves are subject: Light waves consisting of photons are deflected by the atoms in the crystal and overlap – like water waves generated by obstacles in a slowly flowing stream. If a sufficient number of the deflected photons can be measured with a detector, a characteristic diffraction pattern or wave pattern is obtained from which the atomic structure of the crystal can be derived.
However, if the scattering of the photons is incoherent, it is as if the obstacles generated their own waves some indeterminate time after absorbing the waves. In this case, the wave pattern far away does not only depend on the structure, but also on these unmeasurable random delays.
But coherent diffraction X-ray imaging also has a problem: "With X-rays, in most cases incoherent scattering dominates, for example in the form of fluorescence, resulting from photon absorption and subsequent emission," explains first author Anton Classen of FAU. "This creates a diffuse background that can not be used for coherent imaging and reduces the reproduction fidelity of coherent methods."
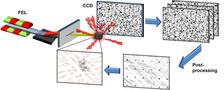
The researchers have now found a way to use exactly this undesirable incoherent radiation background to enhance the imaging with their novel technique. "In our method, the incoherently scattered X-ray photons are not recorded constantly over a longer period of time, but in time-resolved short snapshots", explains FAU Professor Joachim von Zanthier. "If the snapshots are analysed individually, the information about the arrangement of the atoms can still be obtained."
The trick is that the light diffraction is still coherent within short sequences. However, this only works with extremely short X-ray flashes with durations of no more than a few femtoseconds – that is, a few quadrillionths of a second. These short X-ray flashes have only become available rather recently with the advent of free-electron lasers like the European XFEL in Hamburg or the LCLS at the US National Accelerator Laboratory SLAC in California.
“Even though the intensities vary from image to image from the same structure, there is a quantity called the intensity correlation which is conserved so that it remains the same every time. This is what is averaged over many pulses and can be used to determine the structure in a fairly standard way,” explains co-author Kartik Ayyer from the group of Henry Chapman at DESY.
"Since the new method uses fluorescence light, a much stronger signal can be obtained than before, which is also scattered to significantly larger angles, which in turn allows to gain more detailed spatial information," explains co-author Ralf Röhlsberger from DESY, who is also a professor at the University of Hamburg. "In addition, filters can be used to measure the light of specific atomic species only. This makes it possible to determine the position of individual atoms in molecules and proteins with a significantly higher resolution compared to coherent imaging using X-ray light of the same wavelength." The method could thus give new impetus to the study of proteins in structural biology and medicine, the scientists hope.
(from DESY News)
Reference: Incoherent Diffractive Imaging via Intensity Correlations of Hard X Rays; Anton Classen, Kartik Ayyer, Henry N. Chapman, Ralf Röhlsberger, and Joachim von Zanthier; „Physical Review Letters“, 2017; DOI: 10.1103/PhysRevLett.119.053401
Source: FAU (German)