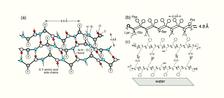
Figure 1: Schematic diagrams. (a) A β-sheet embodying antiparallel set of β-strands interlinked by N-H···O=C bonds floating on the water surface. The hydrophobic groups X emerge from the water surface; groups Y, which are hydrophilic, point into the water. (b) Two peptide β-strands, separated by the N-H···O bonding distance of 0.48 nm, arranged antiparallel. Each CONHCH unit is depicted by a slanted line. (c) Cyclic β-strand dimer of the peptide viewed normal to its plane, along the peptide N-H···O=C direction.
Atalia Birman1, Kristian Kjaer2, Yehiam Prior3, Iftach Nevo4, Leslie Leiserowitz1
1. Department of Materials and Interfaces, Weizmann Institute of Science, 76100 Rehovot, Israel
2. Max-Planck Institute of Colloids and Interfaces, Am Mühlenberg 1, 14476 Potsdam-Golm, Germany, Niels Bohr Institute, University of Copenhagen, DK-2100 Copenhagen, Denmark
3. Department of Chemical Physics, Weizmann Institute of Science, 76100 Rehovot, Israel
4. Department of Chemistry, Aarhus University, DK-8000 Aarhus C, Denmark
Published as: “Laser-Induced Alignment of Self-Assembled Films of an Oligopeptide β-Sheet on the Water Surface”, Angew. Chem. Int. Ed. 122, 2404–2407 (2010).
A pulsed infrared laser beam was used to align oligopeptide molecules at the air-water interface. The oligopeptide was designed to form a cyclic β-strand dimer in a volatile solution. After spreading the solution onto the water surface, illumination with linearly polarized laser light during solvent evaporation induced formation of an aligned crystalline film, whereas circularly polarized laser light did not.
Two-dimensional (2D) crystals at the air-water interface may be obtained by spreading a volatile solution of amphiphilic molecules onto the water surface. During solvent evaporation the amphiphiles may self assemble into monolayer crystals, randomly oriented about the water surface normal, yielding “2D powders” [1]. Our goal is to develop a new approach to induce molecules to self-assemble into aligned 2D crystals at various interfaces, such as air-liquid or liquid-solid.
Peptides composed of alternating hydrophobic and hydrophilic residues will tend to adopt on the water surface a conformation with the hydrophobic groups above the water surface and the hydrophilic below, resulting in a β-strand [2]. The β-strands can interlink by N-H···O bonds with a 0.48 nm repeat distance to form 2D crystalline β-sheets (Fig. 1a). The undecapeptide molecule synthesized here, Pro-Lys-Phe-Glu-Phe-Ser-Phe-Lys-Phe-Glu-Pro (Fig. 1b), is similar to the peptide Pro-Glu-(Phe-Glu)4-Pro, which is known to form a β-sheet monolayer on water surface [3]. The idea is that the undecapeptide molecules will form cyclic β-strand dimers (Fig. 1c) through acid-base Glu-Lys salt bridges in the volatile solution, so that eventually a β-sheet bilayer may form when the solution is spread on top of the water surface.
Infrared reflection absorption spectroscopy indicated that a β-sheet thin film structure was achieved for the selected peptide after spreading the volatile solution onto water surface. Furthermore, grazing incidence X-ray diffraction (GIXD) measurements were carried out at beamline BW1 at DORIS III to study the β-sheet structure in “2D powder” form. These measurements with the film of peptide molecules in a volatile solution floating on water showed two Bragg peaks (Fig. 2e), confirming the existence of the β-sheet structure but as a molecular monolayer (Fig. 1b).
In another experiment the peptide solution floating on water was irradiated with infrared light. For the illumination we used a coherent, linearly-polarized 1.064 μm beam from a Nd:YAG Q-switched pulsed laser (40 mJ/pulse, 50 pulses/sec, 10 ns pulse duration, beam diameter FWHM 3 mm). Illumination was perpendicular to the water surface of a cell onto which we deposited the undecapeptide solution to achieve 80% coverage of peptide. Exposure to the laser beam was for five minutes until the solvent had evaporated. Immediately thereafter the formed film was transferred via horizontal attachment to a Si(111) wafer coated with n-octadecyltrichlorosilane (OTS) giving the Si surface a hydrophobic character.
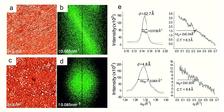
An atomic force microscopy (AFM) topography image (Fig. 2a) of the films created with infrared illumination displays rods oriented along the same diagonal direction. The tendency for alignment is also reflected in the Fourier Transform (FT) pattern (Fig. 2b). The FT lobes are highly anisotropic in shape, perpendicular not only to the mean direction of the rods in the AFM image but also to the direction of polarization of the laser electric field. However, there was an uncertainty of ~15° in relative azimuth angle of the Si wafer with respect to the polarization direction of the laser beam. Results from different samples provided conclusive evidence that the laser illumination with linearly polarized IR light induces alignment persisting over macroscopic distances, while circularly polarized light does not induce preferred orientation (Figs. 2c,d). The average film thickness, derived from the profile of the AFM image, is ~2.3 nm, corresponding to a molecular bilayer (Fig. 1c), as designed.
The approach described here provides a route for thin film engineering, additional means to monitor crystal nucleation and derive structural information of crystal films. In contrast to globular proteins, fewer membrane proteins have been obtained as 3D crystals, although some do form “2D crystalline powders” on water [4]. Therefore, the method described here might provide a solution to the problem of membrane protein crystallization. Since membrane proteins comprise α-helices, which have also been aligned by linearly polarized laser light [5], it may be possible to create aligned 2D crystals of membrane proteins for structural characterization via GIXD.
| References | ||||||||||
|
| Contact information |
|
Leslie Leiserowitz |
| Further Information |