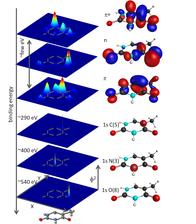
Molecular orbital schemes and electron densities for core-level and valence electronic states in thymine.
Free electron laser facilities provide unique opportunities to probe ultrafast dynamics. Their wide spectral range allows for element specific spectroscopy at the L- and K-edges of many important elements. For us, the K-edges of oxygen, nitrogen and carbon are of most importance as these elements are key ingredients of most organic molecule but L edges of heavier atoms such as sulfur can be similiarly enriching.
Element sensitive spectroscopy means, the core level binding energies of different elements have an appreciable energy difference and are clearly separable. The K-edges of C,N, and O are separated by more than 100 eV.
The image shows binding energies of C. N and O 1s electrons. In addition, the tight binding potential forces the 1s states to be localized in a narrow range of a few picometers around the atom. The left column shows a cut through the orbital charge density of the molecular orbitals – the 1s orbitals are visible as ‘needles’ in the blue background. The valence orbitals, in contrast, are delocalized all over the molecules, as the typical surface plot of the orbitals in the right column shows.
Any transition from 1s to a valence level therefore is highly sensitive on the electronic structure at this particular atom. This is in stark contrast to valence-valence electron transitions.
Our research covers different methods focused primarily on electron spectroscopy. Time-resolved NEXAFS spectroscopy allows us to directly adress electronic states during the relaxation [1] while time-resolved x-ray photoelectron spectroscopy provides access to the valence charge flow [2]. Auger-Meitner spectroscopy gives insight into nuclear degrees of freedom [3,4]. Molecules of interest are currently nucleobases and their thionated analogs as well as photoswitches such as azobenzene.
For our studies at FLASH in Hamburg, our home-built apparatus URSA-PQ is our working horse. We also use the facilities at LCLS, XFEL and FERMI for our experiments.
References
[1] Wolf et al., Nat. Commun. 8, 29 (2017)
[2] Mayer et al., Nat. Commun. 13, 198 (2022)
[3] McFarland et al., Nat. Commun. 5, 4235 (2014)
[4] Lever et al., J. Phys. B: At. Mol. Opt. Phys. 54, 014002 (2020)