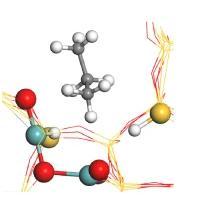
The figure shows the approach of a propane molecule (C atoms in grey) to the postulated binuclear Zn catalyst as the first step of the catalytic cycle. Detail from the figure at the bottom (Credit: From original publication licensed under CC BY 4.0).
The economic and environmental importance of catalysis research can hardly be overestimated. Basically, almost any commercial product nowadays has been in direct or indirect contact with some kind of catalyst during the production process. The need for more sustainable products is a challenge for the chemical industry since many products are still made of fossil sources, like e.g. oil. Due to their high stability, the introduction of functional groups into short-chain alkanes, is a challenging task. For a chemistry based on these compounds, it is mandatory to develop new, environmentally friendly and inexpensive catalysts based on earth-abundant elements.
The non-oxidative dehydrogenation of propane gas to propene (PDH) is a commercial alternative to the oil-based cracking processes used today. Propene is an important ‘platform chemical’ with a global annual production of about 100 million tons. Today's industrially applied PDH technologies utilise Cr- or Pt-containing catalysts. However, the toxicity of Cr(VI) compounds and the limited availability, high costs and usage of harmful chemicals like chlorine to redisperse Pt species during the regeneration of the Pt containing catalyst are the downsides of these technologies.
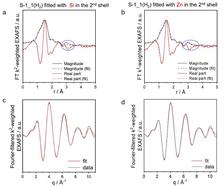
Recently, researchers from the Leibniz-Institut für Katalyse (LIKAT) in Rostock (Germany), together with China University of Petroleum in Beijing, the Shanxi University in Taiyuan (both China) and the Karlsruhe Institute of Technology (KIT) in Karlsruhe (Germany) developed new, inexpensive, environmentally friendly and easy-to-manufacture catalysts for the PDH reaction. Fundamentals required for tailored catalyst design and preparation were also elucidated. Relevant parts of the study, which was published in the journal 'Nature', are based on X-ray absorption fine structure spectroscopy (XAFS) experiments at the PETRA III beamline P65.
Starting point of the study was the observation that a physical mixture of ZnO and a special non-acidic hierarchically porous silicate (silicate-1) support exhibited a promising catalytic performance under industrially relevant conditions. The high propene yield achieved by using this particular catalyst was unique among several tested combinations. Profound knowledge about the nature of the active centres in a catalyst, their formation and fate under reaction conditions are mandatory prerequisites for the improvement of existing catalysts. A comprehensive characterisation of a catalyst and understanding of its functioning is only possible by using a combination of different analytical methods as well as mechanistic and kinetic studies. Analytical methods like XAFS-spectroscopy using synchrotron radiation are required to investigate the relation between structure and catalytic performance.
In the present study, a number of ZnO-based catalysts were prepared using different support materials. The catalytically active Zn-containing sites were generated through a reaction of Zn0 formed from ZnO above 500°C under reducing conditions with OH groups on the surface of the support. After the synthesis step the catalytic performance of the prepared materials was tested under industrially relevant conditions. The conversion rate, selectivity for propene and longevity, was found to be at least as good as an analogue of commercially used Cr-containing catalyst.
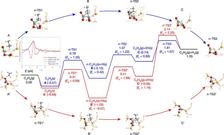
The project leader, E. Kondratenko from LIKAT, said “We combined analytical methods including Electron Paramagnetic Resonance, Diffuse Reflectance Fourier-Transform Infrared Spectroscopy and X-ray Absorption Spectroscopy with kinetic tests, Temporal Analysis of Products with isotopic tracers and Density Functional Theory calculations to unravel the structure of the active sites and molecular details of the PHD reaction.” The XAFS results showed clearly that the active species in the discovered ZnO-derived catalyst is a binuclear ZnOx unit which is formed during the activation process.
The combined analytical and theoretical results demonstrated that silanol (Si–OH) defects located within the micropore structure in zeolites or metal oxides are crucial for the formation of the active Zn-containing species. They determine the structure which is relevant for catalyst activity and product selectivity in the non-oxidative dehydrogenation of alkanes. The concentration of such defects can be adjusted in a controlled manner through the method of support preparation.
This study can pave the way to a purposeful creation of active species and to the establishment of fundamentals relevant for efficient C–H bond activation in various alkanes. Catalysts prepared according to this approach might also find their application in other Zn-catalysed reactions.
Original publication:
Zhao, D., Tian, X., Doronkin, D.E. et al., In situ formation of ZnOx species for efficient propane dehydrogenation, Nature (2021). DOI: 10.1038/s41586-021-03923-3