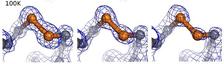
Radiation induced changes in 2Fo-Fc electron density maps of a disulfide bridge of an insulin molecules at 100 K and doses of 9 MGy, 34 MGy, and 60 MGy.
Highly brilliant X-ray sources and electron microscopes open the possibility to obtain structural information with a previously unimaginable level of detail. Such high resolution structure determinations with crystallographic or microscopic techniques require extremely high X-ray or electron doses causing significant damage. Unfortunately radiation damage is an inherent and unavoidable part of any diffraction or imaging experiment using ionizing radiation. Radiation damage alters and subsequently destroys the sample and drastically limits the applicability of these methods.
In the case of macromolecular crystallography radiation damage can be followed by the decay of the Bragg intensity and lattice deterioration, e.g. increase of the crystal mosaicity and the unit cell volume.
Ionizing radiation is known to form different kinds of radicals in the sample. Whereas for pure water the radical species are very well known, much less is known about the X-ray induced radical species in biological and organic molecules with and without solvent. Recently, we could identify hydrogen abstraction from aliphatic carbon atoms as the main radiation induced chemical reaction taking place in organic samples. The hydrogen radicals subsequently combine to form H2 having a drastically lower density than the surrounding crystalline material. This space requiring process leads to undesirable sample deformation and finally ends in a breakdown of the sample.
In future experiments we will further investigate these processes with a combination of X-ray diffraction and techniques such as Infrared and Raman spectroscopy. In addition we plan to combine electron paramagnetic resonance (EPR) and X-ray irradiation to study the lifetime of different radical species as function of temperature and solvent content.
Theoretical Monte Carlo simulations conducted in our group show that, under certain geometrical conditions, the photo electrons generated by inelastic interaction with X-rays, can leave the sample. Since most of the chemical damage arises from energy dissipation of electrons, this effect should reduce the damaging effect of X-ray irradiation and allow the collection of more information from a given sample. We are trying to prove these findings experimentally, and to develop experimental techniques to benefit from these effects.