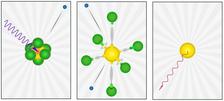
The decay path: (left) SF6 molecule hit by 2.5 keV photon, creating a 1s hole in the sulfur atom (yellow). (middle) Auger cascade relaxation emits electrons causing dissociation. The charge deficit is carried by outgoing fluorine atoms (green). (right) The sulfur atoms retains its electrons in a neutral 2p excited state and emits a 150 eV photon. (Credit: Joseph Nordgren (Uppsala University))
An international collaboration, including researchers from the Fritz Haber Institute (FHI) of the Max Planck Society, has shown in a study the formation of neutral sulfur atoms during the decay of sulfur hexafluoride (SF6) under high-energy X-ray exposure. This study, using advanced synchrotron radiation techniques, provides new insights into the complex interactions of X-rays with matter, essential for scientific and technological advancements. The experiments were carried out at the PETRA III beamline P04.
In 1978, scientists Joseph Nordgren and Hans Ågren at Uppsala University discovered a spectral anomaly in the molecule SF6. The nature of this anomaly remained a mystery for over four decades. The research team revisited this anomaly using advanced techniques developed specifically for this study. In the experiment at PETRA III, SF6 molecules were exposed to hard X-rays that excited sulfur's innermost electron shell, initiating a cascade of electronic decays, leading to complete molecular dissociation and neutral-sulfur-atom formation.
The team, led by Joseph Nordgren (now emeritus professor at the Uppsala University, Sweden), Oksana Travnikova (CNRS, France), and Florian Trinter (FHI Berlin), employed synchrotron radiation to selectively study the soft X-ray emissions of intermediate states. Advanced theoretical calculations were also used to interpret the soft X-ray emissions observed at specific energies which could not be attributed to molecular fragments (e.g., sulfur bound to fluorine atoms) or charged sulfur states. These calculations provided crucial insights into the complex molecular response to deep inner-shell ionisation of sulfur in SF6.
It was determined that, despite the ejection of multiple electrons during the decay cascade, neutral sulfur atoms were formed. This finding is surprising and unexpected given the strong electron-attracting nature of fluorine atoms. The research highlights the intricate electron density redistribution during the decay process and underscores the power of modern X-ray techniques in solving long-standing scientific puzzles.
This research not only resolves a decades-old mystery but also advances our understanding of molecular dynamics under X-ray exposure. The findings have significant implications for both scientific research and practical applications ranging from manipulation of materials to medical imaging and therapy.
Reference
O. Travnikova, F. Trinter, M. Agåker, G. Visentin, J. Andersson, L. Kjellsson, I. Ismail, N. Velasquez, D. Koulentianos, M. Harder, Z. Yin, J. Söderström, T. Marchenko, R. Guillemin, O. D. McGinnis, H. Ågren, S. Fritzsche, M. Simon, J.-E. Rubensson, J. Nordgren, Neutral Sulfur Atom Formation in Decay of Deep Core Holes in SF6, Phys. Rev. Lett. (2025), DOI: 10.1103/PhysRevLett.134.063003