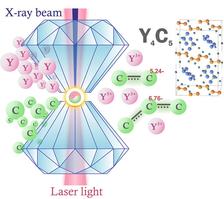
Schemtic drawing of the reaction in the diamond anvil cell (Figure courtesy: T. Fedotenko and L. Dubrovinsky (Bayreuth University)).
The main feature of organic chemistry is the exceptional variety of compounds that have arisen due to the ability of carbon atoms to combine with each other/bind together in unlimited quantities, forming molecules in the form of clusters, limited and unlimited in length chains and cycles. An even greater variety is achieved due to incorporation of oxygen, nitrogen and metals into carbon chaines or clusters. Moreover, the phenomenon of isomerism, if molecules with the same composition have a different structure, additionally increases the variety of organic compounds. Up to now, more than 10 million compounds are known, and their number increases by up to 300,000 annually.
Despite this richness of organic chemistry, the main structural motifs of the "carbon" framework in compounds are limited and repeated. It is believed that the possible variety of carbon fragments in organometallic compounds and metal carbides is also restricted and specified by the organic chemistry rules. Most of the heatherto known metal carbides contain the simplest carbon anions: isolated carbon atoms C4-, dimers [C2]2- and linear [C3]4-units which have the same carbon skeleton as methane, acetylene, and allene, respectively.
High pressure alters these bonding patterns in carbides, leading to new compounds with unusual structural units and interesting properties. Therefore, the compression of these substances might enable exploring the catenation of carbon in more detail. Ab initio-structure search predicts the formation of unusual metal carbides with exotic [C4], [C5] units and [C6] rings, graphitic carbon sheets, and a number of structural transitions. Still, in such predicted carbides, the arrangement of carbon atoms in carbon anions is consistent with those known in organic or metal-organic chemistry. Thus, until recently, it was believed that even under high pressure the carbon entities in metal carbides are limited in possible C-C bond orders and charges of carbon anions.
This paradigm has changed when the new yttrium carbide was synthesised and studied by scientists from Bayreuth University and DESY.
The chemical reaction of yttrium and paraffin oil at pressures of ~50 GPa and temperatures of ~2500°C led to the synthesis of a previously unknown polymorph of yttrium carbide, orthorhombic γ-Y4C5. Reaction products were characterised by synchrotron single-crystal X-ray diffraction performed at beamline P02.2 of PETRA III. The carbon atoms in the γ-Y4C5 crystal structure form [C2] dumbbells and nonlinear [C3] trimers with the bending angle of 134.4(1)°. Usually, [C3] units in known carbides are linear or almost linear with bending angle of 170-180° that is in consistence with the organic analog—allene. A smaller bending angle of about 134° in novel γ-Y4C5 is indeed unique. Density functional theory based charge distribution analysis revealed non-integer charges of carbon units, [C2]5.24- and [C3]6.76-which can be explained by the delocalisation of the electrons donated by Y on the partially filled antibonding π* molecular orbitals of the [C2] and [C3] units. This specific charge distribution results in unusual C-C bond orders of 1.38 and 1.31, respectively, and corroborates with previously never observed bending of the [C3] units.
The findings published in Physical Review Letters demonstrate that carbon polymerisation under high pressure can drastically change the commonly known arrangement of carbon atoms in metal carbides. New chemistry is expected to result in unusual electronic, magnetic, optical properties of carbides, thus opening perspectives for synthesis of a whole class of novel materials.
Original publication:
Novel High-Pressure Yttrium Carbide γ-Y4C5 Containing [C2] and Nonlinear [C3] Units with Unusually Large Formal Charges, A. Aslandukova, A. Aslandukov, L. Yuan, D. Laniel, S. Khandarkhaeva,T. Fedotenko, G. Steinle-Neumann, K. Glazyrin, N. Dubrovinskaia, and L. Dubrovinsky, Physical Review Letters (2021), DOI: 10.1103/PhysRevLett.127.135501