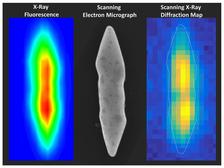
A gold nanoparticle in the light of X-ray fluorescence (left), under the electron microscope (center) and as a scanning X-ray diffraction microscopy image (right). The latter reveals the arrangement of the atoms inside the particle. It is 0.0016 millimeters long. (Credit: DESY, Abhisakh Sarma)
Gold nanoparticles can be better catalysts if their atoms are packed differently from the conventional arrangement. This alternate inner structure leads to different inherent properties. At PETRA III an Indian-German team of scientists has elucidated the inner composition of a gold nanoparticle with mixed crystal structures. The results not only help to better understand the catalytic properties of these nanoparticles but may also aid the production of even more effective catalysts. The team led by Milan K. Sanyal from the Saha Institute of Nuclear Physics in India, Giridhar U. Kulkarni from the Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR) in India, and Christian Schroer from DESY reports the results in the current issue of the journal ACS Nano of the American Chemical Society.
Gold nanoparticles (Au NPs) have a wide spectrum of potential applications due to their unique properties, which are, in many ways, different compared to their bulk form. Although gold in bulk form is poorly active as a catalyst, in the nano form, it exhibits outstanding catalytic activity, especially in redox reactions, such as in the catalytic low-temperature oxidation of carbon monoxide to carbon dioxide, in the oxidation of alcohols, or in selective epoxidation reactions.
“Recent progress of synthetic chemistry enabled us to synthesize a new generation of gold nano-crystals,” reports first author Chaitali Sow from JNCASR. The uniqueness of these crystals is that they have mixed crystal structures, composed of both, the conventional face-centered cubic phase fcc and the non-fcc phases body-centered tetragonal bct and body-centered orthorombic bco. The non-fcc part of the crystallite can be tuned to as high as 85 per cent. “Recent studies reveal that non-fcc gold appears to be a significantly better catalyst than fcc gold”, reports Kulkarni. “Most interestingly, the catalytic properties are found to differ strongly between the fcc and non-fcc phases of gold, despite the relatively small differences in the interatomic bond length,” explains co-author Abhisakh Sarma from DESY.
To better understand the origin of the enhanced catalytic properties, the knowledge about the spatial distribution of the different phases inside the non-fcc gold nano-crystal is essential. The crystallites are few hundred nanometers thick and the conventional high-resolution transmission electron microscopy, which has been generally used to study the occurrence and distribution of unconventional lattice phases in nano-crystals can not be used due to the low penetration depths of the electrons. To elucidate the phase arrangement within the gold nanoparticles, the team used the advanced X-ray techniques available at PETRA III, as the X-rays have high penetration depth and can measure the inner structure.
At the DESY NanoLab, the gold nanocrystals were pre-selected and marked with the focused ion beam instrument prior to the X-ray investigation. “A platinum containing marker material was used that permits to re-locate individual gold nanocrystals with ease,” says Thomas F. Keller from the DESY NanoLab. “The study enabled us to understand the growth direction of individual crystal structures and the corresponding overall crystallites,” explains Sanyal. “The reported X-ray diffraction microscopy technique of representative single crystallites will be useful to investigate the growth-mechanism of similar multiphase nanometer- and micrometer-sized crystals.”
Scanning X-ray diffraction microscopy at PETRA III beamline P06 revealed the nature of the diffracting regions within a bipyramidal (pointy at both ends) gold crystallite that was roughly 1360 nanometres (nm) long and 230 nm thick. A nanometre is a millionth of a millimeter. “The data analysis showed that the body of the crystallite is mostly composed of non-fcc phases whereas the fcc phase resides within its tips,” reports Sarma. “The microscopy also revealed that the non-fcc phase is strained to the extent of roughly five per cent with respect to the fcc phase in the crystallite volume, despite of which the crystallites are stable at ambient conditions for years,” adds Sow. Additionally, the two halves of the bipyramidal lattice are not symmetrical, but twisted along the crystal length by about six degrees.
The investigation shows a path to increasing the non-fcc part of gold nanocrystallites even beyond 85 per cent to further boost their catalytic properties. An in-depth understanding of the distribution of fcc and non-fcc phases in the body and in the tips of the crystallite requires further investigations. Therefore, future studies will focus on combining scanning X-ray diffraction microscopy (SXDM) with a technique called surface-enhanced Raman scattering (SERS) which can map out the catalytic sites at various locations of a microcrystallite to further explore the special gold crystals at the atomic level.
Reference:
Unraveling the Spatial Distribution of Catalytic Non-Cubic Au Phases in a Bipyramidal Microcrystallite by X-ray Diffraction Microscopy; Chaitali Sow, Abhisakh Sarma, Andreas Schropp, Dmitry Dzhigaev, Thomas F. Keller, Christian G. Schroer, Milan K. Sanyal, and Giridhar U. Kulkarni; ACS Nano, 14, 8 (25.8.2020); DOI: 10.1021/acsnano.0c02031