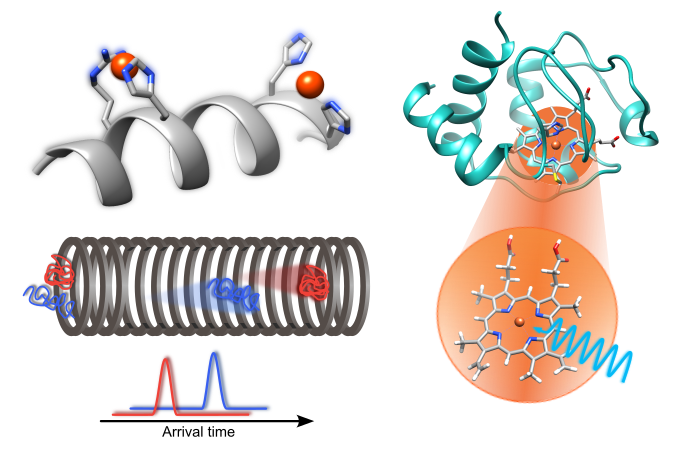
Metalloproteins are proteins containing metallic ions as cofactor or embedded in organometallic molecules such as metalloporphyrins. Metalloporphyrins (MPs) themselves are composed of a porphyrin ring coordinating a metal ion in the ring’s cavity center. Their particular electronic structure makes MPs ideally suited for a number of applications, from biological functions to the usage in electronic devices. The high reactivity of the metalloproteins is intertwined with the oxidation state and spin configuration of the metal ions and their tridimensional structures are likewise affected by the metal localization.
The electronic structure and functional activity of metalloproteins are determined by the type of the metal, its oxidation and spin state as well as axial ligands around the metal center. To understand their properties, it is hence of great importance to probe the local electronic structure of the metal center. Using the electrospray ionisation technique, the study of the intrinsic properties of metal-containing peptides and proteins or MPs can be achieved by conducting studies in the gas phase, i.e. in an ambient-free and controlled chemical state. Here, we apply NEXAMS and quantum-mechanical restricted active space calculations to investigate the electronic structure of the metal-active sites.