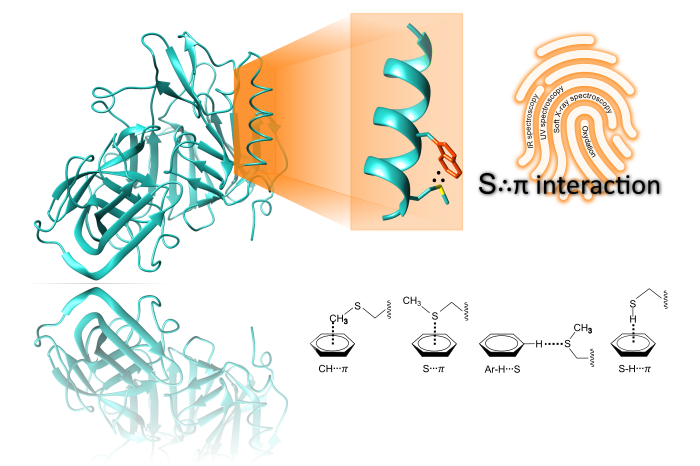
Among the many properties to be unraveled, non-covalent interactions in biomolecules are of particular interest as they are, noteworthy, involved in the proteins' structure stability and molecular recognition.
Our approach toward characterization of the S∴π interaction is to investigate custom-made peptides using action spectroscopy and conformational selection methods. This research focuses on understanding the structural orientation of sulfuric and aromatic groups, recording the change in the vibrational and electronic signatures due to the interaction, and evaluation the effects on the dissociation processes in presence of an S∴π interaction. One main focus is to perform NEXAMS across the carbon K-edge and sulfur L-edge which helps to evaluate this interaction in various peptide configurations. Theoretical absorption spectra computed via DFT/ROCIS help to infer the orientational preferences of sulfur atoms with different aromatic rings. Additionally, the project incorporates multiple spectroscopic approaches, including UV, IR, and ion mobility spectrometry, to comprehensively understand the S∴π interaction's stability and its modifications through sulfur oxidation.