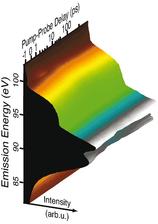
Figure 1: In our experimental setup, a silicon sample is excited with a 400 nm (3.1 eV) optical pulse. With a synchronised 117 eV pulse from FLASH, the electronic structure is probed at various times after excitation through ultrafast X-ray emission spectroscopy.
Martin BeyeA, Florian Sorgenfrei, William F. SchlotterB, Wilfried Wurth, Alexander FöhlischA
Universität Hamburg, Institut für Experimentalphysik and Center for Free-Electron Laser Science, Luruper Chaussee 149, 22761 Hamburg, Germany
A. Present address: Helmholtz-Zentrum Berlin für Materialien und Energie, Institute for Methods and Instrumentation in Synchrotron Radiation Research G-I2, Wilhelm-Conrad-Röntgen-Campus, Albert-Einstein-Str. 15, 12489 Berlin, Germany
B. Present address: SLAC National Accelerator Laboratory, 2575 Sand Hill Road, Menlo Park, CA 94025, USA
Published as: “The liquid-liquid phase transition in silicon revealed by snapshots of valence electrons”, Proc. Natl. Acad. Sci. USA 107, 39, 16772–16776 (2010).
Microscopic models for the „anomalies of water“ are still lacking an experimental proof, although anomalous thermodynamic behaviour is common for a class of matter that forms tetrahedral networks - like water, diamond or silicon. Their phase diagrams are very rich, but the exploration of large areas has been limited mostly to theoretical studies. And yet, remainders of those experimentally unaccessible areas contribute to the properties at standard conditions. For example a possible explanation for the anomalies is based on the existence of a liquid-liquid phase transition in the supercooled region. With the combination of ultrashort optical laser pulses and soft X-ray pulses from FLASH, we study the melting dynamics of silicon in detail. We find two distinct melting steps separated by several picoseconds, which we attribute to the disputed liquid-liquid phase transition.
Silicon is one representative out of a whole class of so-called tetrahedral network formers, spanning from the IV- and III-Vsemiconductors like silicon, germanium, indium-antimonide to silica, carbon and water [1]. These materials commonly share a phase diagram with many stable phases, including the ability to form glasses and different liquid phases [2]. Many predictions about the existence of a liquid-liquid phase transition have been made in theory [3]. However, so far no experiment has been able to directly access the relevant temperatures and pressures, because the phases of interest are metastable with nanosecond lifetimes [4].
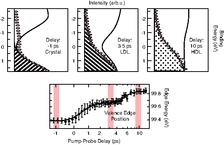
The optical excitation of electrons in a silicon sample from the valence to the conduction band, induced by a femtosecond pulse from a 400 nm laser, can generate enough energy in the form of “hot electrons” to guide the temperature and pressure through the relevant part of the phase diagram. While the excited electrons lose their energy to the lattice via electron-phonon coupling, as quantified previously [5], the temperature and pressure in the sample increases and the sample melts into a liquid state. We identified the characteristic electronic structure of the liquid state with X-ray emission spectroscopy using synchronised soft X-ray pulses from FLASH. The solid-liquid phase transition takes place on a timescale faster than one picosecond, which cannot be resolved in our experiment. Surprisingly, the liquid phase initially does not show the spectral signature of the expected, thermodynamically stable high-density liquid (HDL), which would have metallic character and a higher density than the crystalline solid.
Instead, the molten silicon is semi-metallic with a gap in the density of states around the Fermi level, which is characteristic for the low-density liquid (LDL) with a density similar to the crystal. Silicon stays in this phase for the first four picoseconds after laser excitation. The explanation for this is twofold: on one hand, the change in density must involve the coordinated motion of several atoms that have to move considerable distances to form bubbles of higher density and voids in between. Therefore the transition to the HDL takes time. On the other hand, the metastable LDL phase lies in a local free energy minimum in configuration space, separated by an activation barrier from the equilibrium HDL state [1]. To overcome this barrier - the latent heat of the first order liquid-liquid phase transition - more energy needs to be transferred from the electronic system to the nuclei. We observed how hot electrons smoothly decayed from their reservoir in the conduction band until, after sufficient heat transfer, the electronic structure changes abruptly to the metallic, gapless state around six picoseconds after the laser excitation.
With our measurements of the electronic structure of silicon after strong laser excitation, we can unambiguously identify a first order liquid-liquid phase transition in silicon and therefore contribute substantially to a long-living debate in literature with implications to other materials forming tetrahedral networks, most notably water. Moreover, we explore time-resolved X-ray emission spectroscopy in general as a highly selective tool, which makes full use of the unique properties of free-electron lasers to study ultrafast dynamics of the electronic structure. In the future, we will extend the use of this technique to study more complex materials and systems, including analysis of transient states during chemical reactions in the liquid phase and catalytic processes on surfaces.
| References | ||||||||||
|
| Contact information |
|
Martin Beye |
| Further Information |