Scheme. 1
Helmut Zepik1, Edna Shavit1, Mao Tang1, Torben R. Jensen2, Kristian Kjaer2, Gérard Bolbach3, Leslie Leiserowitz1, Isabelle Weissbuch1, Meir Lahav1
1 Department of Materials and Interfaces, Weizmann Institute of Science, 76100 Rehovot, Israel.
2 Materials Research Department, Risø National Laboratory, 4000 Roskilde, Denmark.
3 Laboratoire de Chimie Structurale Organique et Biologique, Université Pierre et Marie Curie, 75252 Paris Cedex 05, France.
Published in: Science, Vol. 295, Issue 5558, pp. 1266-1269, February 15, 2002
Many organic molecules are chiral: Their S and R (left- and right-handed) enantiomers are non-superimposable mirror images of each other. Chemical reactions by default yield racemic mixtures consisting of 50:50 R and S enantiomer. However, in Nature proteins consist of only S-amino acids and RNA and DNA contain only R-sugars. This break of symmetry may have occurred through the amplification to 100% of a slight initial imbalance. In this study, an amphiphilic alpha-amino acid derivative was designed such that, organized in 2D crystalline self-assemblies at the air-water interface, its racemic mixtures could be converted into homochiral oligopeptides by a "lattice-controlled" polymerization reaction...
Theories on the emergence of the homochiral biopolymers of life at prebiotic times suggest the involvement of enantioselective reactions starting from heterochiral mixtures of alpha-amino acid and nucleic acid precursors (1-7). Polymerization reactions of racemates in isotropic media would lead, however, to formation of polymers comprising a random sequence of left (S)- and right (R)-handed repeat units in a binomial distribution (8). Thus, the probability of obtaining oligomers with homochiral sequence will become negligible with increasing length (9-11). A possible way to obtain oligopeptides of homochiral sequence from racemic mixtures would be through the self-assembly of the precursor molecules into ordered architectures followed by lattice-controlled reactions.
Two-dimensional (2D) self-assemblies formed at the water surface provide an ideal medium for the performance of stereospecific and enantioselective chemical reactions because (i) they promote effective molecular ordering, (ii) they permit substantial molecular flexibility, and (iii) they allow access to the ordered, flexible molecules by other reagents from the aqueous subphase (12-14). Moreover, with the recent advent of grazing incidence x-ray diffraction (GIXD) using synchrotron radiation, it became possible to determine the structure of the crystalline components of these assemblies at the molecular level, providing an important tool for the design of new architectures and control of their reactivity (15).
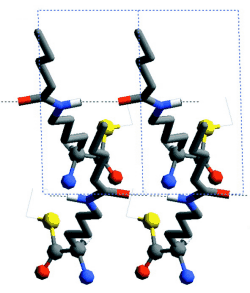
Racemic mixtures of amphiphilic molecules at interfaces can self-assemble into 2D crystallites of three types: (i) racemic compounds in which both enantiomers are packed together, (ii) enantiomorphous conglomerates involving segregation of the enantiomers (16), and (iii) enantiomerically disordered solid solutions. Here, we focus on activated alpha-amino acid derivatives that self-assemble into 2D crystallites as racemic compounds that, upon polymerization, yield oligopeptides with an enhanced homochiral (isotactic) sequence, as shown, in principle, in Scheme 1A. This concept is illustrated with the polycondensation of racemic Nepsilon-stearoyl-lysine-thioethylester (C18-TE-Lys). For comparison, we present the related racemic gamma-stearyl thioglutamic acid (C18-T-Glu), which self-assembles such that the two enantiomers are almost randomly distributed within the crystallites, thus forming a solid solution. Polymerization of this system yielded oligopeptides of almost random distribution of the R and S repeat units (Scheme 1B). The packing arrangements of the 2D crystallites on the water surface were determined by GIXD, and the composition of the oligopeptides was analyzed by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) with enantio-labeled samples.
A different type of process involves an amplification of chirality, starting from a low enantiomeric imbalance of the monomers. Oligopeptides exhibiting a single handedness can be prepared by self-assembly of chiral nonracemic mixtures of the monomers into separate racemic and enantiomorphous crystallites. This amplification process can be achieved efficiently provided that polymerization, within the racemic 2D crystallites, occurs between molecules of opposite handedness (Scheme 1C) yielding heterochiral (syndiotactic) oligopeptides, as illustrated for gamma-stearyl-glutamic acid-N-carboxyanhydride (C18-Glu-NCA). The enantiomer in excess will yield the homochiral oligopeptides.
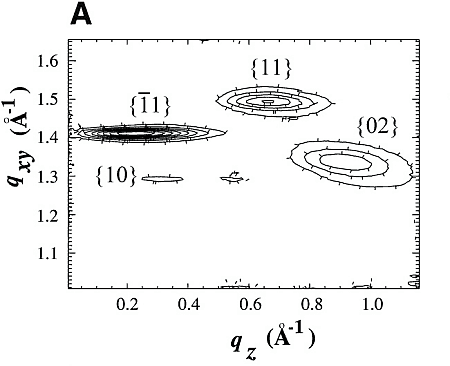
Chloroform solutions of enantiomeric or racemic C18-TE-Lys (17) in the form of their trifluoroacetate salts were spread on the water surface for 70% monolayer coverage. One of the enantiomers of racemic C18-TE-Lys incorporated perdeuterated hydrocarbon chains (labeled Sd or Rd), leaving the other unlabeled (Rh or Sh). The GIXD patterns measured from the enantiomeric (R) or (S) and racemic (R,S) phases of C18-TE-Lys on water (Fig. 1, A and B) are substantially different. The derived unit cell of the enantiomeric phase is centered pseudo-rectangular (a = 4.89 Å, b = 9.43 Å, gamma = 93.9°) containing two molecules whose chains are tilted by 35° from the surface normal in a direction 15° off the b axis. The GIXD data from the racemic mixture yield a similar unit cell (a = 4.94 Å, b = 9.04 Å, gamma = 91.4°) containing two molecules whose chains are tilted by 33° from the surface normal, but in a direction almost parallel to the b axis. This result, coupled with the observation that the dimensions of the unit cell projected along the chain axis (ap = 4.9 Å, bp = 7.5 Å, gammap approx.= 90°), is fingerprint evidence of herring-bone chain packing achieved by pseudo-glide symmetry relating two molecules of opposite handedness. The molecular packing arrangement, determined to near atomic resolution by x-ray structure factor-constrained least-squares analysis (18, 19), is shown in Fig. 1C.
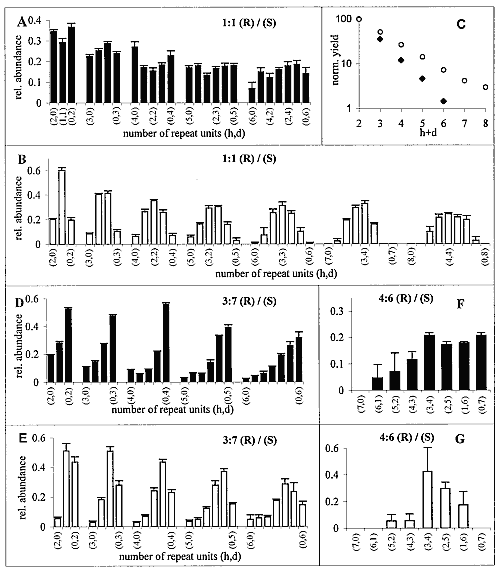
Polycondensation of racemic C18-TE-Lys was achieved by injection of I2/KI (20), or AgNO3 (21) solutions into the aqueous subphase at 20°C or 1°C. After 2 to 3 hours of reaction, the samples were collected from the surface and analyzed by MALDI-TOF MS (22). As a result of the enantioselective labeling of only the S enantiomer with deuterium (see above), we obtain a mass difference of 35 mass units per monomer (23, 24). Consequently, the number of R and of S monomer units could be determined for each diastereoisomeric oligopeptide. The total ion abundance of the different oligopeptides, normalized to that of the dipeptide product (Fig. 2C), exhibits an exponential decrease with increasing length of the oligopeptides. The distribution of the different diastereoisomers for each oligopeptide length (Fig. 2A) reveals a clear trend toward enhanced formation of homochiral Rh and Sd peptides vis-à-vis their heterochiral counterparts. For comparison, Fig. 2B shows the distribution of oligopeptides obtained from the polycondensation of racemic C18-T-Glu (25). This racemate self-assembled into an enantiomerically disordered solid solution (Scheme 1B) that, upon polycondensation, yielded essentially a random distribution of oligopeptides (Fig. 2B), providing a reference system more realistic than the theoretical calculation for a random system (8).
A comparison between Fig. 2, A and B, shows a distinct bias for the C18-TE-Lys system to form homochiral diastereoisomers, labeled (h,0) and (0,d), at the extremities of each distribution for di- to hexa-oligopeptides.
The enhanced concentration of the homochiral C18-TE-Lys oligopeptides (Fig. 2A) is in agreement with the packing arrangement of the racemic monomer (Fig. 1C). The amino group of one molecule is appropriately oriented and in close proximity to a carbonyl group of a nearest-neighbor homochiral molecule (~5.0 Å) to react and form a peptide bond (Fig. 1D). In contrast, the amine and carbonyl groups of two heterochiral molecules related by glide symmetry are less appropriately aligned for a nucleophilic attack (Fig. 1E).
Next, we describe amplification of homochirality starting from chiral nonracemic mixtures in systems where a phase separation occurs: The racemic fraction forms a racemic compound and the enantiomer in excess assembles into enantiomorphous 2D crystallites (26). This concept is illustrated first for 7:3 and 6:4 (S:R) mixtures of C18-TE-Lys. A distinct enhancement of the homochiral fraction of the Sd oligopeptides [labeled (0,d) in Fig. 2, D and F] relative to that of the heterochiral molecules (h,d) was obtained especially for penta- to heptapeptides (27) as compared, once again, with the products obtained from the corresponding mixtures of C18-T-Glu with random polycondensation (Fig. 2, E and G).
In the C18-TE-Lys system, however, the formation of the oligopeptides with homochiral sequence of the minor component [labeled (h,0) in Fig. 2, D and F] cannot be prevented because of the intrinsic formation in the racemic phase of both homochiral Rh and Sd oligopeptides (Scheme 1A). This drawback can be circumvented (see above) in systems where the reaction in the racemic crystallites occurs primarily between heterochiral molecules (Scheme 1C) and the enantiomer in excess polymers of a single handedness. This concept is illustrated for C18-Glu-NCA.
The GIXD pattern of enantiomeric C18-Glu-NCA (Fig. 3) on water yields a pseudo-rectangular unit cell (a = 5.52 Å, b = 8.62 Å, gamma = 92.5°) with a molecular arrangement shown in Fig. 3C. The chains are tilted by 34.8° from the surface normal in a direction 15° off the a axis. Injection of the nickel acetate catalyst into the aqueous subphase induces a reaction that could be monitored by GIXD. A minor, yet important, change occurs in the unit cell that becomes rectangular (a = 5.54 Å, b = 8.58 Å, gamma = 90°), in keeping with chains of the polymerized film tilted by 34° from the surface normal in a direction parallel to the a axis.
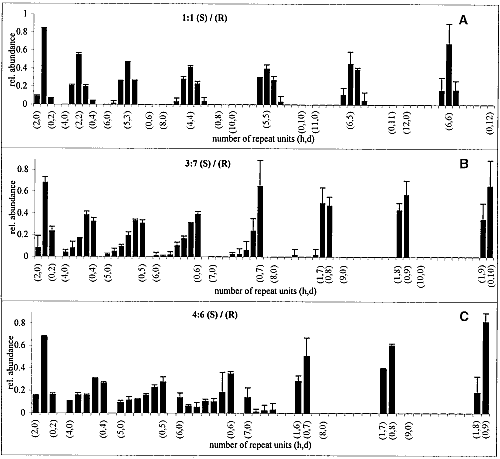
The GIXD patterns of racemic C18-Glu-NCA on pure water and on aqueous solution containing a catalyst are similar to that shown in Fig. 3B, namely, no change in the diffraction pattern was observed during the reaction. According to the proposed packing arrangement of the racemic monomer (Fig. 3D), a lattice-controlled polymerization occurs between heterochiral molecules related by glide symmetry (Scheme 1C), which is strongly supported by the MALDI-TOF MS analysis (Fig. 4A). This histogram shows the formation of heterochiral oligopeptides in concentrations beyond the random distribution obtained from C18-T-Glu (Fig. 2B). When starting from chiral nonracemic mixtures of 3:7 and 4:6 (S:R) compositions, the generated short oligopeptides are rich in heterochiral diastereoisomers, whereas the longer oligopeptides are rich in homochiral sequences of single handedness (Fig. 4, B and C). Oligopeptides 9 or 10 units long obtained from the 3:7 (S:R) mixtures consisted only of compositions1S:8R and 0S:9R or 1S:9R and 0S:10R. Similarly, the 4:6 (S:R) mixtures yielded oligopeptides of only 1S:7R and 0S:8R or 1S:8R and 0S:9R compositions (Fig. 4, B and C) (28).
In conclusion, we have demonstrated that polymerization within 2D crystallites composed of racemic compounds appropriately packed can lead to the enhanced generation of oligopeptides with homochiral sequences through lattice-controlled reactions between molecules of the same handedness. Furthermore, an efficient amplification of homochiral oligopeptides starting with monomers of low enantiomeric imbalance was accomplished by segregation into racemic and enantiomorphous phases followed by reactivity. This process takes advantage of the differences in the packing arrangement and kinetics of polymerization within the racemic and enantiomorphous crystalline phases of the monomers, thus introducing nonlinear effects (29) and resulting in libraries of oligopeptides of different lengths and handedness.
Scenarios describing the appearance of chiral amino acids under prebiotic conditions such as irradiation of racemates with circularly polarized light (30), stochastic crystallization processes (31), or amino acids that had reached Earth by way of meteorites (32) are always of low enantiomeric imbalance. The mechanism of amplification of chirality described here might be relevant in converting such mixtures of amino acids into oligopeptides of single handedness.
Finally, it has been proposed that self-assemblies of amphiphilic molecules have played a ubiquitous role at early stages of evolution in the formation of primitive membranous "minimum protocells" (33, 34). The present results suggest that ordered self-assembled architectures of appropriate amphiphiles in aqueous media or on the surfaces of minerals might have also been instrumental in the formation of the first optically active biopolymers.
| References |
|
[1] F. C. Frank, Biochim. Biophys. Acta11, 459 (1953) |
| Contact information |
|
Kristian Kjaer |