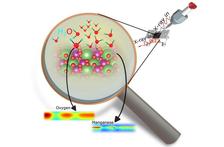
X-ray methods developed at the DESY high-flux sources make it possible to chemically selectively investigate the active layer during water splitting - an example of the possible applications of the DESY sources to precisely characterise modern energy materials. (Credit: DESY)
Those who understand down to the atomic level what happens when energy is converted in solar cells—switching elements of computer chips or reactors for the production of hydrogen—can optimally improve them so that they function as quickly, economically, and for as long as possible. This requires methods to observe the processes at the atomic and even subatomic level on timescales of picoseconds or even femtoseconds. These are quadrillionths of a second. “Extremely brilliant X-ray sources, such as those we operate with PETRA III, FLASH, and European XFEL at DESY, provide the light intensity to achieve the necessary spatial, temporal and chemical resolution,” says Simone Techert, head scientist of the Chemical Structural Dynamics group at DESY and Professor of Ultrafast X-ray Physics at the University of Göttingen.
More efficient electrolysis
Take catalytic water splitting by electrolysis, for example: This process is used to produce hydrogen while generating oxygen at the same time. This requires a catalyst that enables and accelerates water splitting - on both the hydrogen and oxygen side. And this is where research is looking for the most efficient candidates. Perovskites are considered particularly promising for oxygen production, as they can be used under low-energy operating conditions and are abundant in nature.
Perovskites are a class of minerals with a special crystal structure that is particularly favorable for the conversion of energy. “The decisive factor for the effect of catalytic water splitting is what transpires at the Helmholtz layer—the interface where water and catalyst come into contact,” says Simone Techert. “With the help of our X-ray methods, especially at PETRA III and with some test experiments at FLASH, we were able to examine this layer more closely and identify the various short-lived chemical species that form during electrolysis. You can actually see how individual atoms of the catalyst interact with water and form short-lived complexes, which in turn can trigger migrations of catalyst atoms on the surface.” According to Techert, the scattered photons of the X-rays can even be used to track subatomic quantum effects when the water molecules lose electrons and transfer them to the catalyst atoms on the surface. This dynamic is surprising: According to theory, a very rigid image of the catalyst surface was previously predicted.
The researchers have now examined both the perovskite catalyst and the reacting water during catalytic water splitting using X-rays. This provides a more accurate, complete picture of oxygen formation and the catalysts can now be optimised.
The fastest possible processors
Another example of the new methods tested for examining energy materials is Ultrafast Transmission Electron Microscopy. In this technique, a material sample is exposed to a flash of light and irradiated with ultrashort electron pulses to see how the material reacts. Kai Rossnagel, a senior scientist at DESY and physics professor at Kiel University who researches quantum materials, has contributed a suitable model material: tantalum disulphide (TaS2). “This crystal sandwich consisting of a layer of tantalum atoms between two layers of sulphur atoms is a kind of Drosophila of material physics,” he says. “Like the fruit fly in biology, it is often used as a reference because it shows so many interesting phase transitions when you change the temperature or shoot it with a flash of light, for example.”
Phase transitions are changes in the arrangement of atoms and electrons due to external influences. The experiments revealed that the flashes of light in TaS2 cause a veritable formation dance of the particles: The electrons redistribute themselves in the crystal lattice of the atoms, forming a new, different superstructure of denser and less dense clouds, “like waves and valleys of electron density, the distribution of which is reminiscent of the shape of an egg carton,” explains Rossnagel.
Under an electron microscope, the two phases appear as lighter and darker spots. This phase transition can be controlled by a flash of light, within a few picoseconds. “This means,” says Rossnagel, “that we are dealing with a possibility for ultra-fast control processes in information technology.” This is because the material also changes its ability to conduct electricity well or less well with the phases.
With the new methods, we can observe exactly how and why this happens so quickly - and possibly transfer the mechanism to our electronic devices at some point.” Processors, such as those in our cell phones, could become up to a thousand times faster and more energy-efficient: “We have a lever for gigantic electricity savings in our hands,” says Rossnagel.
The new methods could also significantly advance the development of quantum materials through to quantum computers. Tiny defects in the quantum material could be detected and corrected in order to counteract the susceptibility of quantum computers to interference.
A whole range of new methods
Both examples—X-ray methods for investigating electrical catalysis and electron microscopy for investigating quantum effects—are part of a whole series of new methods, some of which are based on work at PETRA III and FLASH. They have been included in a recently published review article summarising the results of the Collaborative Research Centre (SFB) “Control of Energy Conversion on Atomic Scales” funded by the German Research Foundation (DFG). In addition to various institutes at the University of Göttingen, DESY was also involved in the SFB.
The bottom line, according to the two DESY researchers Techert and Rossnagel, is that the new high-resolution microscopy and spectroscopy methods open up analytical possibilities that can decisively advance the energy revolution. And this is urgently needed, says Techert: “Because we need to achieve the energy revolution as quickly as possible in order to keep climate change in check.”
(from DESY News)
Reference:
Kai Rossnagel and Simone Techert et al, "Advancing Energy Materials by In Situ Atomic Scale Methods", Advanced Energy Materials, 2025, DOI: 10.1002/aenm.202404280