3D remodeling of the tumor spheroid (Credit: Original publication)
Nanotechnology is significantly advancing medicine. Tiny, specially designed particles deliver active substances into diseased cells or have a healing effect themselves. To ensure that this happens as safely and effectively as possible, the behaviour of the nanoparticles after entering a cell must be studied in detail.
In a new study, an international research team led by Prof. Wolfgang Parak from the Centre for Hybrid Nanostructure (CHyN) at the University of Hamburg, Gerald Falkenberg from DESY and Carlos Sanchez-Cano from the Donostia International Physics Centre (Spain) investigated which methods are best suited for observing how nanoparticles of different sizes penetrate spheroids and distribute themselves there. Spheroids are three-dimensional cell aggregates used in cell culture to simulate tissue or tumours. Cancer served as an example of many diseases for which nanoparticles may offer a therapeutic option.
The investigations were carried out at CHyN and at the PETRA III beamline P06. This beamline is specialised in X-ray microscopy and enables various investigation methods. ‘PETRA III offered the right combination of high radiation intensity, strong focusability and extensive experience of the beamline staff in handling the various methods for measuring and reconstructing 3D objects,’ says Sanchez-Cano. ‘This enabled us to image the samples in high resolution and carry out many measurement series in a relatively short time.’
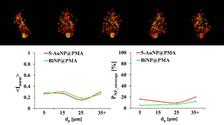
The researchers added two types of nanoparticles of different sizes to spheroid samples to observe how far the particles penetrate into the cell cluster models and how they distribute themselves there. They used gold and bismuth nanoparticles ranging in size from 1 to 100 nm – not least to test the influence of size on penetration. ‘Logically, smaller particles generally penetrate more easily and more deeply,’ says Sanchez-Cano. ‘But there are certain sizes that penetrate better than others. And of course, we want to know which ones those are.’
However, the primary aim was to evaluate and compare the suitability of different methods for investigating the penetration of nanoparticles into cells. The researchers tested variants of optical fluorescence microscopy, flow cytometry, inductively coupled plasma mass spectrometry (ICP-MS), transmission electron microscopy (TEM) and X-ray fluorescence microscopy at PETRA III. It became apparent that each method has its advantages and disadvantages and that they should rather be used in combination to complement each other.
TEM offers excellent resolution down to one nanometre and below, thus showing all details. However, it requires intensive preparation of the samples: the tissue must be dehydrated and stained. In addition, the sample must be cut ultra-thin, as the penetration depth is very low. ‘And because the sample is prepared so intensively, the distribution of the nanoparticles does not necessarily correspond closely to reality,’ says Gerald Falkenberg, coordinator of beamline P06 at DESY. ‘This raises the question of the transferability of the results to living tissue.’
This is different with optical fluorescence microscopy: Here, the tissue remains as it is, and instead the nanoparticles are labelled with a fluorescent marker so that they can be observed live as they penetrate the tissue. Optical light is easy to handle, but it only penetrates a maximum of about 100 micrometres into the tissue before it scatters too much. The resolution is much lower than with TEM, so individual nanoparticles cannot be detected. Another disadvantage is that the marker can influence the behaviour of the nanoparticle, as it itself has a certain size, which is particularly significant in the case of small particles.
‘X-ray fluorescence microscopy is a good complement,’ says Sanchez-Cano. ‘It requires little or no preparation or labelling and images the nanoparticles themselves. The radiation penetrates deep into the tissue, as we know from X-rays. However, it is problematic to use it on living tissue because of the potential damage caused by the intense radiation.’
The bottom line is that it depends on what exactly you want to investigate about the behaviour of medical nanoparticles under which circumstances – depending on this, you should choose a different combination of observation methods. However, the study showed one thing very clearly: ‘PETRA III already offers very good possibilities for investigating such things – it is not without reason that we preferred it to other X-ray sources,’ says Sanchez-Cano. ‘However, we are eagerly awaiting the realisation of PETRA IV, the update of PETRA III to a fourth-generation X-ray source. This would allow us to take such investigations to a whole new level in terms of precision and speed.’
PETRA IV will achieve 100 times greater radiation intensity than the current facility for microscopy experiments and allow for significantly finer beam sizes. This not only enables higher image resolution, but also means considerably faster scanning: ‘Each individual experiment with a specific configuration took about 24 hours in the current study,’ says Sanchez-Cano. ‘With PETRA IV, we could reduce that to less than an hour.’ DESY physicist Falkenberg adds that the novel Compton microscopy technique would also allow the same or even better quality results to be achieved with far less radiation damage. ‘And other experiments that require high resolution and long irradiation times will become possible for the first time.’
PETRA IV will also be even more versatile: ‘Not only can we see where the nanoparticles are located in the cell, but we can also determine their chemical and physical properties at the same time using spectroscopy and diffraction,’ explains Falkenberg. This is already possible with PETRA III, but it takes much longer and carries the risk of falsifying results, for example due to radiation damage.
The versatile P06 beamline will be replaced by three more specialised beamlines at PETRA IV. This will enable even more sophisticated studies. ‘It is important that in a PETRA IV experiment, the samples can be prepared and measured cryogenically, i.e. frozen,’ says Falkenberg. ‘This brings the sample closest to its living state, avoids preparation artefacts and reduces radiation damage. We are already starting to use such cryogenic measurement setups.’
In addition, the bioimaging experiment at PETRA IV will combine such investigations with holographic methods, which will significantly accelerate the three-dimensional imaging of objects. ‘With PETRA IV, we can enter a whole new dimension,’ says Falkenberg. ‘And that applies not only to biomedical research, but to many other areas as well. In the chemical industry, for example, we will optimise catalysts and find new ones that enable different and more sustainable production processes and products.’
Reference:
"Size-dependent penetration depth of colloidal nanoparticles into cell spheroids", Dingcheng Zhua, Dennis Brücknerc, Martin Sosnioka, Marvin Skibaa, Neus Feliud, Marta Gallegoe, Yang Liua, Florian Schulza, Gerald Falkenberg, Wolfgang J. Paraka, Carlos Sanchez-Canof, ScienceDirect (2025), DOI: 10.1016/j.addr.2025.115593