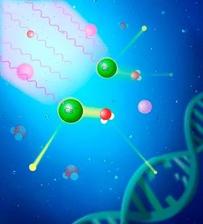
Resonantly excited calcium ions (green) in an aqueous environment undergo resonant Auger intermolecular Coulombic decay (RA-ICD), thereby ionizing a neighboring water molecule. In this process, potentially harmful low-energy electrons are emitted, which could damage nearby DNA molecules (Credit: Figure reused under CC BY 4.0 from original publication / D. Bloß et al. JACS 2025).
Important evidence of a special DNA-damaging mechanism has been successfully demonstrated for the first time under realistic conditions – providing a crucial basis for potential cancer therapies. An international research team led by Dana Bloß from the Institute of Physics at the University of Kassel has confirmed a central molecular process in aqueous solution, a process that could also occur in the human body. Low-energy electrons (LEEs), which are known to damage DNA structures, were released in a targeted manner. Until now, this mechanism had only been observed in the gas phase.
The study was carried out in an international collaboration with research groups from Sorbonne Université (Paris, France), the Fritz-Haber-Institut (Berlin), Uppsala University, the MAX IV Laboratory (Lund, Sweden), Technische Universität Dortmund, and Universität Heidelberg and was published in the Journal of the American Chemical Society (JACS).
The detection of the mechanism was made possible by a combination of advanced electron spectroscopy methods and high-energy X-rays from synchrotron light sources. Using the electron coincidence technique, the team identified the underlying process: a complex molecular mechanism in which harmful electrons are generated. It is called resonant Auger intermolecular Coulombic decay (RA-ICD). These experiments were performed at the FlexPES beamline at the MAX IV Laboratory in Lund.
In combination with high-resolution electron spectroscopy, the released electrons could be identified and their formation in the immediate molecular environment confirmed. This showed that electrons with an energy of 15-25 electron volts are specifically generated in calcium ions in water – precisely in the range known to be particularly effective at causing DNA damage. These high-resolution Auger spectra were recorded at the PETRA III soft X-ray beamline P04, which is suitable for liquid-jet electron spectroscopy.
The RA-ICD mechanism was already theoretically predicted and experimentally observed in 2014, and its potential medical applications were also discussed at that time. The underlying idea is to activate certain marker elements that are specifically introduced into malignant tissue, for example by means of resonant excitation with X-rays. The X-rays interact almost exclusively with the marker atoms, releasing specific electrons in their immediate vicinity in a targeted and efficient manner, which can damage DNA. In contrast, the surrounding atoms and molecules of healthy tissue remain largely transparent to the radiation, minimizing collateral damage. Unlike conventional X-ray therapy, where radiation is distributed widely throughout the tissue, this approach would enable localized and minimally invasive treatment.
"Even though our study is still basic research, we were able to show that harmful electrons in water can be generated specifically at defined points," explains Bloß, who conducts research in experimental physics with synchrotron radiation. "This is an important step towards a possible application of the process in more precise and tissue-sparing therapy concepts." However, she emphasizes that there could still be a long way to go before such applications can be used in treatment.
Reference:
D. Bloß et al., Site- and Energy-Selective Low-Energy Electron Emission by X-rays in the Aqueous Phase, J. Am. Chem. Soc. (2025), DOI: 10.1021/jacs.5c06436