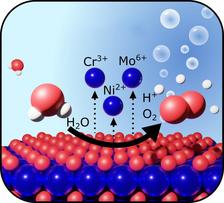
OER induced degradation: Passivity breakdown of Cr-containing alloys upon anodic polarization is traditionally believed to be caused by oxidation of stable Cr3+ oxide to soluble Cr6+ species leading to fast metal dissolution. By using multimodal in situ synchrotron measurements of highly-alloyed Ni-base alloys, we report a new passivity breakdown mechanism, namely oxygen evolution reaction (OER) induced metal dissolution occurring at lower anodic potentials. (Figure: From the original publication licensed under the Creative Commons Attribution License 4.0 (CC BY))
Corrosion-resistant metallic materials are needed for applications such as rocket engines, energy systems, and the chemical industry. An alloy of nickel (Ni), chromium (Cr) and molybdenum (Mo) is often used here. A new study at PETRA III and MAX IV in Sweden now shows that under certain conditions this alloy corrodes in a previously unknown way.
Buildings, means of transport, works of art and musical instruments - metallic materials are used almost everywhere in society. What limits their lifespan is often corrosion, where a material corrodes and dissolves through chemical reactions. Costs associated with corrosion damage amount to about three percent of the world's GDP each year. In addition, the traditional steel-making process generates high amounts of carbon dioxide (CO2) emission.
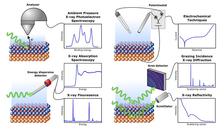
In this study published in the scientific journal Advanced Materials, a research team from Sweden and Germany, led by Lund University and KTH Royal Institute of Technology in Stockholm, studied an alloy of Ni, Cr and Mo known for its corrosion resistance. Researchers from Alleima in Sandviken, Malmö University, Swerim AB in Kista, MAX IV as well as from Hamburg Universität and DESY were also involved. Through accelerated electrochemical tests at the synchrotron lightsources MAX IV and PETRA III, the researchers found that the alloy corrodes in a very different way than other Cr-containing alloys such as stainless steels. These measurements were partly carried out at the "Swedish Materials Science Beamline” P21.2 and beamline P64 at PETRA III and the DESY NanoLab.
Thanks to a combination of five experimental techniques using synchrotron radiation, the researchers gained insights into the corrosion process occurring at the surface of the material during accelerated tests. They could prove that the water splitting reaction plays an important role in the corrosion process which was neglected previously. The splitting of water into oxygen and hydrogen is activated by the catalytic function of the Mo ions and it is not just an additional reaction that occurs alongside the corrosion reactions. It also seems to drive and even trigger corrosion reactions. This effect can cause problems for the industry, since simple laboratory electrochemical tests will not be able to distinguish between the water splitting and corrosion reactions. Based on this knowledge, industry can develop new protocols for corrosion testing in the future. This, in turn, can lead to the correct assessment of which material should be used for different applications. A better corrosion control strategy, would save both money and natural resources and lead to a more sustainable use of resources.
The demonstrated experimental approach is also relevant to the investigation of the green hydrogen production, a field where intensive research is being performed worldwide. Therefore, the methodology of this study also can be used to gain a detailed knowledge about how electrode materials in fuel cells and electrolysers work, and why they are stable or degrade under operation conditions.
(Partly from Lund University news, by J. Joelsson - published 14 Sept. 2023)
Original Publication:
The Oxygen Evolution Reaction Drives Passivity Breakdown for Ni–Cr–Mo Alloys, A. Larsson, A. Grespi, G. Abbondanza, J. Eidhagen, D. Gajdek, K. Simonov, X. Yue, U. Lienert, Z. Hegedüs, A. Jeromin, T. F. Keller, M. Scardamaglia, A. Shavorskiy, L. R. Merte, Jinshan Pan, and Edvin Lundgren, Adv. Mater. 2023, DOI: 10.1002/adma.202304621