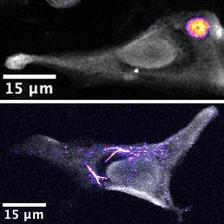
Depending on the route of administration, the intracellular distribution of the selenium-based drug changes. When non-biodegradable polymers are used as the building blocks of the capsules, the selenium remains in the container and is not released (upper picture). The situation is different when amino acid and sugar-based vehicles are used which are digested by the cell and result in intracellular redistribution of the drug (lower picture). Cells are shown in grey while selenium is pseudocoloured from blue to yellow, depending on the concentration. (Credit: DESY, Marvin Skiba)
How can cancer drugs be delivered safely to their destination? An international team of researchers has been using the X-ray source PETRA III to test a technique for visualising how a drug is distributed inside tumor cells. In the future, this approach could help to develop more targeted and hence more effective cancer therapies. The working group has presented its findings in the journal Advanced Functional Materials.
Some anticancer drugs present a special challenge. They do not dissolve easily in the blood or they break down too quickly and because of this they are unable to reach the site where they are needed: the tumor. Researchers have come up with an ingenious strategy to overcome this: they enclose the drug in a molecular capsule. On being administered, this medication taxi makes its way through the body. Once it reaches the tumor, the capsule dissolves and releases the drug.
The only trouble is that it is difficult to observe how well this strategy is working. How do the drug capsules find their way into the tumor cells? And do they actually release the drug inside them? To answer these questions, researchers have until now had to label the drugs using special dyes. When a laser beam is shone at these, they light up like signal lamps and reveal the distribution of the drug inside a cell.
This method has its drawbacks, however. The markers are usually similar in size to the drug molecules themselves, and this can distort the readings. “It’s as if you were trying to track a fish through the ocean by fitting it with a transmitter that is as big as the creature itself,” explains Marvin Skiba, a PhD student in Wolfgang Parak’s group at the University of Hamburg’s Centre for Hybrid Nanostructures. “In that case, it’s doubtful whether the fish would move around in the same way as it would without the transmitter.” It would be helpful, therefore, to have a way of seeing the drug inside the medication taxi without having to label it with a dye.
One promising approach is X-ray fluorescence, a technique that can detect minute traces of a chemical element. The principle is straightforward. “When an X-ray beam strikes a sample, it excites the elements in it,” explains DESY physicist Gerald Falkenberg. “The excited atoms want to shed this energy quickly by emitting X-ray quanta. We use detectors to capture these quanta.”
The crucial point is that every element emits a different “X-ray colour”, thereby leaving its own distinctive fingerprint. The X-ray beam scans the sample line by line, creating a map of the elements. This requires a very powerful, narrow X-ray beam, such as the one generated by DESY’s X-ray source PETRA III at beamline P06.
To determine the suitability of this method for studying drugs transported in medication taxis, Skiba and Falkenberg’s team focused on a compound containing the element selenium, a potential therapeutic for treating tumors. “We enclosed the compound in a variety of different microparticles,” explains Marvin Skiba. “We then injected these into a cell culture and used X-rays to track how the selenium was distributed in the cells.”
They found that shortly after being injected, the micrometre-sized medication taxis were still clearly visible in the X-ray images. After 24 hours, though, they had disappeared. Only the selenium could still be seen, along with its distribution within the cell. “From this we conclude that the taxis entered the cell and were digested and destroyed there,” reports Skiba. “This means that the selenium compound was released and was able to spread inside the cell.” As a scientific control, the experts also enclosed the drug in a more robust capsule, one which the cell was unable to break down, and repeated the experiment. They observed that, although the capsules entered the cells, they held onto their cargo – figuratively speaking, the doors of the taxi didn’t open.
“Certain chemical techniques may be able to measure the selenium levels in the cell more precisely,” says Gerald Falkenberg, “but our method allows us to map its distribution with high spatial resolution. It visualises the distribution of selenium in the cell, showing whether it is in the cell nucleus or outside it.” The selenium compounds studied served primarily as models. In principle, the method could also be applied to other drugs more relevant to cancer research. “Many of the drugs that have now been approved are based on metal compounds,” says Skiba. “With our method it should be possible to see where they actually end up in the body.”
The researchers now want to optimise their technique and investigate, for example, how the medication taxis behave in larger tissue structures. To do so, they intend to expand their method. Up to now, they have scanned their samples in two dimensions. In the future, they want to do this in 3D as well and obtain depth information about the distribution of a drug. Studies involving living cells are also being considered, but so far the experiments have only worked successfully in dead cells.
In the longer term, experiments conducted at PETRA IV may also be promising. DESY’s planned next-generation X-ray source will provide significantly more intense and narrowly focussed radiation than today’s PETRA III facility. “On the one hand, this means that the measurements can be performed much faster,” says Falkenberg. “On the other hand, the image resolution could be markedly increased.” As a result, the images of the drugs spreading around the cell would be much sharper.
(partly from DESY News)
Reference:
Exploring the Intracellular Distribution of Se Compounds Delivered by Biodegradable Polyelectrolyte Capsules Using X-Ray Fluorescence Imaging, Marvin Skiba, Rebeka R. Reszegi, Yalan Huang, Sathi Roy, Jili Han, Dennis Brückner, Carlos Sanchez-Cano, Ying Zhao, Moustapha Hassan, Neus Feliu, Gerald Falkenberg, Wolfgang J. Parak, „Advanced Functional Materials“, 2024, DOI: 10.1002/adfm.202408539