Figure 1: (A, B, C, D) Changes of different diffraction data quality indicators with dose as function of temperature for cubic insulin (black) and elastase crystals (red): (A) mean intensity decay per MGy, (B) normalized unit cell expansion, and (C) normalized crystal mosaicity of. (D) Increase of the Rfree values with dose obtained from the structure refinements of the intensity datasets. Each cross in graphs A, B, C, and D corresponds to data from one crystal. The solid line connects mean decay values at each temperature with its standard deviation.
A. Meents1,2, S. Gutmann2,4, A. Wagner3, and C. Schulze-Briese2
[1] now at: HASYLAB - DESY, Germany.
[2] Swiss Light Source (SLS) - Paul Scherrer Institut, Switzerland.
[3] Diamond Light Source, United Kingdom.
[4] Present adress: Novartis Institutes for Biomedical Research, Novartis Pharma AG, CH-4056 Basel, Switzerland.
Published as: “Origin and temperature dependence of radiation damage in biological samples at cryogenic temperatures”, PNAS, vol. 107, no. 3, 1094-1099 (2010).
Radiation damage to biological samples has become a major limitation for experiments at brilliant 3rd generation synchrotron sources such as Petra III and free electron lasers. Radiation damage leads to sample deterioration and hence drastically limits the applicability of experimental techniques employing high doses of radiation [1, 2]. Cryo-cooling of samples down to 100 K or even lower can reduce but not prevent radiation damage [3].
Until now radiation damage to biological samples has not been completely understood. By combining different experimental techniques we have identified radiation induced C-H bond cleavage and the subsequent formation of hydrogen gas as the main source of radiation damage at cryogenic temperatures. The apparent contradictions in results previously discussed in the literature are finally resolved by this novel and conclusive mechanism for radiation damage in biological samples at cryogenic temperatures. In addition the commonly applied data collection temperature of 100 K has been proven not to be the optimal choice. Radiation damage is reduced by up to a factor of four when experiments are conducted at temperatures around 50 K instead of 100 K. Further lowering the temperature to 30 K and below did not result in any further reduction of radiation damage.
X-ray crystallography, small angle X-ray scattering, and monochromatic and white beam irradiation experiments have been applied to identify the basic mechanism of radiation damage. Cubic insulin and elastase crystals have served as model systems for the crystallographic component of this work. X-ray intensity data were collected at beamline X06SA at the Swiss Light Source (SLS) in Villigen with a Pilatus 6M detector [4]. Data were collected for temperatures between 5 K and 160 K. The decay of the recorded Bragg intensities, changes of the unit cell volume and crystal mosacity, and the increase of the R(free)-value from structure refinements with dose were chosen as radiation damage parameters [5,6]. The changes of each of these parameters with dose as function of temperature are shown in figure 1. Interestingly for all of these parameters a local extremum or a parameter jump is observed at 30 K, most prominently for the unit cell volume increase.
Small angle X-ray scattering from cubic insulin crystals was performed to directly follow this radiation induced disordering process. The integrated diffuse scattering signal of cubic insulin crystals as a function of dose is shown for different temperatures in figure 2. For all temperatures a two regime behaviour is observed. At low doses the signal increases moderately in a linear fashion with dose. After reaching a certain 'critical dose' the signal starts to grow exponentially indicating a fast disorder process. This 'critical dose' increases linearly with decreasing temperature demonstrating an ideal gas behaviour, e.g. at 50 K the critical dose is twice as much as at 100 K.
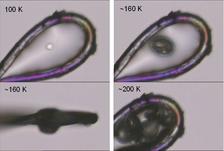
Warming of irradiated protein crystals above their glass transition temperature [7] leads to a gas release (figure 3). To identify the nature of this gas, white beam irradiation experiments of different organic compounds were carried out. In all cases hydrogen gas was identified as being the major gaseous component of the radiolysis experiments. Smaller organic fragments such as methane or ethane were only minor products indicating that direct C-C bond cleavage is not the major cause of radiation damage. A quantitative analysis of the gas amount formed upon irradiation with monochromatic X-rays showed no temperature dependence, except for the case of water where an increase in gas formation was observed at lower temperatures.
Combining these results leads us to the conclusion that radiation induced cleavage of the C-H bond and the subsequent formation of hydrogen gas is the major cause for radiation damage in biological samples at cryogenic temperatures. Lowering the data collection temperature from 160 K to 50 K significantly reduces the radiation damage, as derived from the lower decay of the Bragg intensities and the slower increase of the R(free)-values with dose (that is, we observe a better preservation of the structure). At these temperatures hydrogen gas is still mobile inside the sample and can accumulate at grain boundaries or leave the crystal. At 30 K the hydrogen gas becomes immobile [8], as expressed in the faster unit cell and mosaicity increase, and leads to unfavourable sample deformation. In the case of crystalline samples these lattice distortions finally result in a faster loss of diffracting power at 30 K.
Additionally, the commonly applied temperature of 100 K has been shown to be suboptimal. Observing the increase of the R(free) value of cubic insulin and elastase crystals, radiation damage is reduced by 74% and 62% respectively, when experiments are performed at 50 K.
These results were mainly obtained from X-ray crystallographic measurements, though we expect them to be valid for other methods employing high doses of ionizing radiation, e.g. X-ray and electron microscopy.
| References | ||||||||||||||||
|
| Contact information |
|
Alke Meents |
| Further Information |