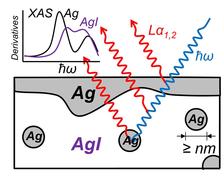
Schematics of the XAS experiment for a soft Ag/AgI system which was proved to contain nanosized crystallites of active metal (Ag) submerged in the AgI electrolyte. (Figure Credit: Authors)
Electrochemical metallization resistive switching memories are current major candidates for replacing the state-of-the-art dynamic random access memory and non-volatile flash memories in future nanoelectronics and information technology, and new encouraging concept for logic and neuromorphic applications are feasible today. Scientists from Forschungszentrum Jülich and RWTH Aachen university investigated the chemical stability of an Ag/AgI interface, where AgI is the thin electrolyte layer between the two electrodes of a memory cell. The XAS measurements were performed at the DORIS III beamline A1 and were published in 'Scientific Reports'.
Dissolution of active metal ions into a host material in the memory cell, which is an essential process for the memory action, unfortunately can degrade the stability of the memory cell, because it can change the transport properties of the host material and disturb the mechanical contact between the active electrode and the electrolyte. To avoid such type of complications, materials with almost minimum possibility for deviation from the stoichiometry such as RbAg4I5 or AgI have been studied nowadays. However, even for AgI thin films, the researchers have observed that thin layer of Ag apparently dissolves into AgI resulting in almost complete loss of electric contact.
One of the obstacles that hinder simple analysis for this interesting system using conventional chemical tools such as photoemission, Auger electron spectroscopy or electron energy loss spectroscopy, is that Ag/AgI is “soft” in that its chemistry or structure can be changed easily under physical or electrochemical stresses (e.g. sputtering or electron beams) which are inevitable for those techniques. Therefore, they used instead a non-destructive way to examine the chemistry of the Ag/AgI system, X-ray absorption spectroscopy (XAS). Another obstacle for the reliable analysis is that it is in principle impossible to distinguish by valence states only, the metallic Ag atoms of the top electrode from those diffused into the AgI electrolyte itself. This was overcome by mining the structural information of the specific Ag atoms additionally with the fine structure analyses. The researchers particularly investigated the evolution in the XAS spectra upon the additional Ag deposition.
Surprisingly, the XAS results showed that there is neither chemical interaction nor any local structural distortion at the Ag/AgI interface despite the high ionic mobility of Ag ions. Simulation results further showed that Ag metal clusters can form in the AgI layer with intermediate-range order at least up to next-next nearest neighbors, suggesting that Ag can permeate into the AgI only in an aggregated form of metal crystallites of a sub-mesoscopic scale.
Original publication: Chemically-inactive interfaces in thin film Ag/AgI systems for resistive switching memories, Deok-Yong Cho, Stefan Tappertzhofen, Rainer Waser and Ilia Valov, Sci. Rep. 3, 1169 (2013). DOI: 10.1038/srep01169